A noncarbonated soft drink contains an unknown amount of citric acid, H 3 C 6 H 5
Question:
A noncarbonated soft drink contains an unknown amount of citric acid, H3C6H5O7. If 100. mL of the soft drink requires 33.51 mL of 0.0102 M NaOH to neutralize the citric acid completely, what mass of citric acid does the soft drink contain per 100. mL? The reaction of citric acid and NaOH is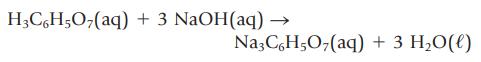
Transcribed Image Text:
H3C6H5O₂(aq) + 3 NaOH(aq) → Na3C6H5O7(aq) + 3 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
First lets calculate the moles of NaOH used in the reaction Given Volume of NaOH solution 3351 mL 0...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Although slow start with congestion avoidance is an effective technique for coping with congestion, it can result in long recovery times in high- speed networks as this problem demonstrates....
-
Write your answer based on the Graph given below. B F E 1. Using Array [Adjacent Matrix] show the memory representation of the following graph. 2. Using Linked List [Adjacent List] show the memory...
-
When organic compounds containing sulfur are burned, sulfur dioxide is produced. The amount of SO 2 formed can be determined by reaction with hydrogen peroxide: H 2 O 2 (aq) + SO 2 (g) H 2 SO 4 (aq)...
-
1. Consider the linear system Ax = b (all integer values) with the notation shown below, 1 -3 -1 -2 - H 5 7 - 2261x11 X2 -4 3 9 16x3 615 LX4- = A X b The element a24 = a has been lost. Assume,...
-
The following data are monthly sales of jeans at a local department store. The buyer would like to forecast sales of jeans for the next month, July. (a) Forecast sales of jeans for March through June...
-
Let a particle P be free to slide radially in a rotating tube as shown in Figure 1.10. Assume the tube is rotating at a constant angular velocity w. What is the inertial velocity and acceleration of...
-
Young Adultmagazine states the following hypotheses about the mean age of its subscribers. a. What would it mean to make a Type II error in this situation? b. The population standard deviation is...
-
The following data were provided by the accounting records of NewFort Limited at year- end, 31 December 20X9: Analysis of selected accounts and transactions: a. Sold plant assets for cash; cost, $...
-
Which of the following is NOT a feature of method 1 of accounting for partnership equity? a. Interest on capital is credited to the partners retained earnings accounts. b. Partners drawings are...
-
Sodium thiosulfate, Na 2 S 2 O 3 , is used as a fixer in black-and-white photography. Suppose you have a bottle of sodium thiosulfate and want to determine its purity. The thiosulfate ion can be...
-
Sodium bicarbonate and acetic acid react according to the equation What mass of sodium acetate can be obtained from mixing 15.0 g of NaHCO 3 with 125 mL of 0.15 M acetic acid? NaHCO3(aq) + CH3COH(aq)...
-
J. Bartley Pools Inc. reported the following in its financial statements for the quarter ended March 31, 2014. During the quarter ended March 31, 2014, Bartley reported Net Income of $5,000 and...
-
4. (15pt) A group of students were asked if they have ever driven after drinking. They also were asked, "How many days per month do you drink at least two beers?" In the following discussion, 7 = the...
-
discuss how might you apply the concepts of Total Quality (TQ) to your personal and work environment. Consider your relations with others and your daily activities interactions with. Share the...
-
Dr. Bernstein wants to expand his radiology practice. Dr. Bernstein is researching various local banks for the best certificate of deposit rate to fund his expansion. One bank is willing to offer him...
-
An airplane is flying with a velocity of 240 m/s at an angle of 30.0 with the horizontal, as the drawing shows. When the altitude of the plane is 2.4 km, a flare is released from the plane. The flare...
-
Katsura Corporation incurred pre - operating costs: Investigatory expenses of $ 1 8 , 0 0 0 New employee training $ 2 5 , 0 0 0 Advertising $ 1 0 , 0 0 0 Land and building for use as a retail store...
-
Refer to Short Exercise S2-3. Which of the transactions of Sandy Lyle, MD, increased the total assets of the business? For each transaction, identify the asset that was increased. In Short Exercise...
-
The first law of thermodynamics is sometimes whimsically stated as, You cant get something for nothing, and the second law as, You cant even break even. Explain how these statements could be...
-
Careful measurements reveal that para-methoxybenzoic acid is less acidic than benzoic acid, while meta-methoxybenzoic acid is more acidic than benzoic acid. Explain these observations.
-
Predict the major product(s) formed when hexanoyl chloride is treated with each of the following reagents: (a) CH 3 CH 2 NH 2 (excess) (b) LAH (excess), followed by H 2 O (c) CH 3 CH 2 OH, pyridine...
-
Predict the major product(s) formed when cyclopentanecarboxylic acid is treated with each of the following reagents: (a) SOCl 2 (b) LAH (excess), followed by H 2 O (c) NaOH (d) [H + ], EtOH
-
-
-
Padie, inc, buzs 80 percentef the outstanding common-stock of siera, Corporaticn on January 1:2021, for $/66,240 cash, At the was only $650000 Also, several weviduat sems on Siera's financial records...
-
On January 1, 2022, Mills Corp. purchased a call option on shares of XYZ stock. Terms of the contract were as follows: Number of shares: 100 Strike price: $260 per share Expiration date: April 30,...

Study smarter with the SolutionInn App


