An unknown solid acid is either citric acid or tartaric acid. To determine which acid you have,
Question:
An unknown solid acid is either citric acid or tartaric acid. To determine which acid you have, you titrate a sample of the solid with aqueous NaOH and from this determine the molar mass of the unknown acid. The appropriate equations are as follows: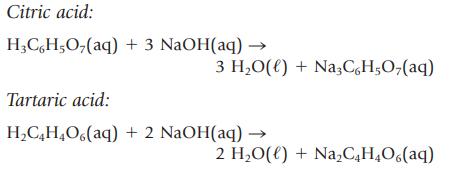
Transcribed Image Text:
Citric acid: H3CH₂O₂(aq) + 3 NaOH(aq) - 3 H₂O(l) + Na3C,H,O,(aq) Tartaric acid: H₂C4H₂O(aq) + 2 NaOH(aq) → 2 H₂O(l) + Na₂C4H₂O6(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine which acid citric acid or tartaric acid you have you can titrate a sample of the solid with aqueous NaOH and then determine the molar mas...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Evergreen company has assets of $500 million and $125 million in liabilities. For the past year, Evergreen earned $150 million and paid out $20 million in dividends. What is the company's return on...
-
Suppose that the stock price S, follows lognormal distribution St= Soe(a-8-0.50) t+oiz Prove that the conditional expectation of lognormal prices, when terminal stock price ST falls below Kis where d...
-
Assume analysts provide the following types of information. Assume no short selling and a risk free-rate of 10%. What is the optimal investment? Expected Return Standard Deviation Asset 1 10% 5%...
-
Why must we recognize and address challenges caused by diversity and work to implement a more inclusive healthcare workforce?
-
Compute the Cpk measure of process capability for the following machine and interpret the findings. What value would you have obtained with the Cp measure? Machine . USL = 100 LSL = 70 Process = 5...
-
A rectangular plate is supported by three cables as shown. Knowing that the tension in cable AD is 195 lb, determine the components of the force exerted on the plate at D. 36 Dimensions in hehos
-
The website for the Bed and Breakfast Inns of North America gets approximately seven visitors per minute (Time, September 2001). Suppose the number of website visitors per minute follows a Poisson...
-
Green Grocery Company employed Jones as its manager. Jones was given authority by Green to purchase supplies and goods for resale and had conducted business for several years with Brown Distributing...
-
During the year the company purchased equipment through the issuance of common stock. The stock had a par value of S200,000 and a fair value of $350,000. The fair value of the equipment was not...
-
To analyze an iron-containing compound, you convert all the iron to Fe 2+ in aqueous solution and then titrate the solution with standardized KMnO 4 . The balanced, net ionic equation is A 0.598-g...
-
You have 0.954 g of an unknown acid, H 2 A, which reacts with NaOH according to the balanced equation If 36.04 mL of 0.509 M NaOH is required to titrate the acid to the second equivalence point, what...
-
Youve recently learned that the company where you work is being sold for $275,000. The companys income statement indicates current profits of $10,000, which have yet to be paid out as dividends....
-
Complete the "Leadership Vision Questionnaire" in Chapter 7 (p176). Reflect on your results and complete the following prompts: Share the results from your questionnaire. Be sure to include the final...
-
1. Prepare el Presupuesto Operacional hasta completar el COGS (70 puntos) La empresa ACCO 295 tiene una venta proyectada de $450,000 Cada unidad se vende $450 Su inventario inicial 300 (costo $125)...
-
Continuing Case 65. Retirement Income Forecast Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that...
-
The partnership of Frick, Wilson, and Clarke has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the following account balances: Cash...
-
Harry and Sally went to a large hardware store and told the salesperson they wanted the cheapest rotating clothesline in stock, provided it would bear a heavy load of washing. The salesperson assured...
-
The manager of Upod, Inc., prepared the balance sheet of the company while the accountant was ill. The balance sheet contains numerous errors. In particular, the manager knew that the balance sheet...
-
The manager of a local convenience store is expanding his line of small toy items. To price these new items, the manager is looking at the prices being charged by competing retailers in his area. For...
-
Which of the following processes is spontaneous? a. The reversible isothermal expansion of an ideal gas. b. The vaporization of superheated water at 102C and 1 bar. c. The constant pressure melting...
-
One joule of work is done on a system, raising its temperature by one degree centigrade. Can this increase in temperature be harnessed to do one joule of work? Explain.
-
Your roommate decides to cool the kitchen by opening the refrigerator. Will this strategy work? Explain your reasoning.
-
Required information The Foundational 15 (Algo) (LO5-1, LO5-3, LO5-4, LO5-5, LO5-6, LO5-7, LO5-8] (The following information applies to the questions displayed below.) Oslo Company prepared the...
-
Required information Use the following information for the Exercises below. [The following information applies to the questions displayed below. Ramirez Company installs a computerized manufacturing...
-
Moving the delivery of services from within the organization to a provider outside the organization is referred to as delivery transfer. outcome sourcing. delivery diffusion. outsourcing.

Study smarter with the SolutionInn App


