Balance the following equations: (a) For the synthesis of urea, a common fertilizer (b) For the reactions
Question:
Balance the following equations:
(a) For the synthesis of urea, a common fertilizer
![]()
(b) For the reactions used to make uranium(VI) fluoride for the enrichment of natural uranium 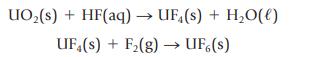
(c) For the reaction to make titanium(IV) chloride, which is then converted to titanium metal 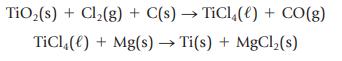
Transcribed Image Text:
CO,(g) + NH,(g) → NH,CONH,(s) + H,O({)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Lets balance the chemical equations a For the synthesis of urea COg 2NH3g NH2CONH2s H2O Explanation ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balance the following equations using the method outlined in Section 3.7: (a) C + O2 CO (b) CO + O2 CO2 (c) C1 + Br2 HBr (d) K + C1O KOH + C1 (e) Mg + O2 MgO (f) O3 O2 (g) C1O2 C1O + O2 (h) N2 + C1...
-
Balance the following equations and write the corresponding ionic and net ionic equations (if appropriate): (a) (b) (c) Ba(OH)-(aq) + HPO 4 (aq )- HCIO4 (aq) + Mg(OH )2 (s)
-
Balance the following equations and write the corresponding ionic and net ionic equations (if appropriate): (a) (b) (c) CH3COOH (aq) + KOH(aq)- .co.(aq) + NaO H (aq) - HNO3(aq ) + Ba(OH)2(aq )-
-
Which one the below does not define "Work role boundaries" of a care worker limits that allow a patient and staff to connect safely in a therapeutic relationship based on patients' needs rules of...
-
Does our discussion of moneys usefulness as a medium of exchange and unit of account suggest reasons why some currencies become vehicle currencies for foreign exchange transactions?
-
There is an infinite wire grid with square cells (Fig. 3.38). The resistance of each wire between neighbouring joint connections is equal to R0. Find the resistance R of the whole grid between points...
-
MIRR Project X costs $1,000, and its cash flows are the same in Years 1 through 10. Its IRR is 12%, and its WACC is 10%. What is the projects MIRR? AppendixlLO1
-
SDC Inc. has established the following standard cost per unit: Materials4 lb @ $4.50 per lb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $18.00 Labor-2hr @ $12 per hr . . . ....
-
4. Record the depreciation expense and any other adjustments related to the vehicle on December 31, 2022. (If no entry is required for a transaction/event, select "No Journal Entry Required" in the...
-
Balance the following equations: (a) For the reaction to produce superphosphate fertilizer (b) For the reaction to produce diborane, B 2 H 6 (c) For the reaction to produce tungsten metal from...
-
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent. (a) CH4(g) + 3 O(g) 2 CO(g) + 2 HO(l) (b) Si(s) + 2 Cl(g) ...
-
What are the quality requirements for suppliers?
-
Centurion Inc. manufactures lighting equipment. It consists of several operating divisions within its business. Division A has decided to go outside the company to purchase materials since Division B...
-
Meta has also reduced its operations, and instead focused on retaining wealth for research and development, as well as increasing shareholder returns...What does this mean for the company's future?
-
Please answer the following question short and simple: Tom Anderson is the controller for Morningside Medical Clinic. At the end of each month, the financial management system used by Morningside...
-
Give a brief general description of the number of degrees of freedom. A. The number of degrees of freedom for a collection of sample data is the number of unique, non-repeated sample values. The...
-
Suppose you are given a data frame df. df = pd.DataFrame({'Click_ID':['A', 'B', 'C', 'D'], 'Count':[100, 200, 300, 400]}) In many data science projects, you are required to convert a dataframe into a...
-
Selected transactions for Thyme Advertising Company, Inc. are listed here. 1. Issued common stock to investors in exchange for cash received from investors. 2. Paid monthly rent. 3. Received cash...
-
1. What are some current issues facing Saudi Arabia? What is the climate for doing business in Saudi Arabia today? 2. Is it legal for Auger's firm to make a payment of $100,000 to help ensure this...
-
Propose a mechanism for the following transformation. Et Me [H,SO] Etw Me -
-
What reagents would you use to prepare each of the following thiols: a. b. c. SH SH
-
Predict the products for each of the following reactions. a. b. c. d. SH 1) NaOH Br 2) Br SNa
-
ething manfactors robbed on the reported to fotoweg tundude for det med and direct later for the year Blandard Standard Standart OL 2 tard Low b.72059.464 Code koton wes 16.000 23.600 tl we feet of...
-
Budgeting A sales budget is given below for one of the products manufactured by the Borders Co January 20,000 units February 35.000 units March April May June 00.000 units 40.000 units 31,000 units...
-
Journalize the following transactions for Matts Carpentry, Inc. (If no entry is required, select "No Entry" for the account titles and enter 0 for the amounts. Credit account titles are automatically...

Study smarter with the SolutionInn App


