Balance the following equations, and name each reactant and product: Data given in Example 3.1 (a) SF4(g)
Question:
Balance the following equations, and name each reactant and product: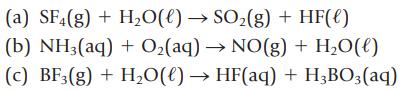
Data given in Example 3.1
Transcribed Image Text:
(a) SF4(g) + H₂O(l) → SO₂(g) + HF(e) (b) NH3(aq) + O₂(aq) → NO(g) + H₂O(l) (c) BF3(g) + H₂O(l) → HF(aq) + H₂BO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a SFg 2HOl SOg 4HFl Reactants Sulfur tetrafluoride gas and water Products ...View the full answer

Answered By

Amit Choudhary
I'm new in this profession regarding online teaching but previously i used to teach students near my college. I am teaching on online platform since last year and got good support from the students. I'm teaching on platforms like chegg and vedantu and also at my home in free time.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balance the following equations, and name each reactant and product: Data given in Example 3.1 (a) FeO3(s) + Mg(s) MgO(s) + Fe(s) (b) AlCl3(s) + NaOH(aq) AI(OH)3 (s) + NaCl(aq) (c) NaNO3(s) +...
-
Balance the following equations and write the corresponding ionic and net ionic equations (if appropriate): (a) (b) (c) Ba(OH)-(aq) + HPO 4 (aq )- HCIO4 (aq) + Mg(OH )2 (s)
-
Balance the following equations and write the corresponding ionic and net ionic equations (if appropriate): (a) (b) (c) CH3COOH (aq) + KOH(aq)- .co.(aq) + NaO H (aq) - HNO3(aq ) + Ba(OH)2(aq )-
-
Which statement about the pass-by-reference is NOT true? a.Every time you pass a reference variable to a method, you also pass the object referred by the refenerece variable to the method. b.In the...
-
Describe the factors to consider before developing an aggregate plan.
-
Single fuel cells such as the one of Example 1.4 can be scaled up by arranging them into a fuel cell stack. A stack consists of multiple electrolytic membranes that are sandwiched between...
-
6. Describe how you can apply the principle, "The people you know the least contribute the most to your network" to the process of a job search. Are you abusing your relationships for personal gain?
-
1. If Lightcos contribution margin is 40 percent, what increase in sales will it need to break even on the increase in fixed costs to hire the new sales reps? 2. How many new retail accounts must the...
-
Q3. You are given the following transactions a. Parent purchased inventory for SR 300 and t sold the inventory to a Sub for SR 400 not yet sold to third party. b. Parent purchased inventory for SR...
-
Write balanced chemical equations for the following reactions. (a) The reaction of aluminum and iron(III) oxide to form iron and aluminum oxide (known as the thermite reaction). (b) The reaction of...
-
Balance the following equations: Data given in Example 3.1 (a) Cr(s) + Cl(g) CrCl3(s) (b) SiO (s) + C(s) Si(s) + CO(g) (c) Fe(s) + HO(g) Fe3O4(s) + H(g)
-
Under the right conditions, the following acid-catalyzed double cyclization proceeds in remarkably good yields. Propose a mechanism. Does this reaction resemble a biological process you have seen?...
-
f. The coordinates of two points A and B are (1, 2) and (5,7) respectively. Find the equation and slope of the line AB. g. Find the rate of change of the area of a circle w.r.t its radius r when r =...
-
1. Sketch the anticipated pattern of cracks on the beam structure shown below. Assume that the structure is adequately reinforced for the load shown, and that the loads are large enough to cause...
-
Estimate the hydrogen consumption required to completely remove the sulfur from a hydrotreater feedstock and to reduce the nitrogen content of the product to 15 ppm by weight. The 48.5 API naphtha...
-
2. Consider the following kinds of information, and suggest the most appropriate data type to store or represent each: Information Suggested Data Type String A person's name A person's age in years A...
-
Steam at 32 MPa, 520C enters the first stage of a supercritical reheat cycle including three turbine stages. Steam exiting the first-stage turbine at pressure p is reheated at constant pressure to...
-
Botany Clothiers reported the following amounts in its 20X6 financial statements. The 20X5 figures are given for comparison. Required 1. Compute Botanys acid-test ratio at the end of 20X6. Round to 2...
-
Evaluate the function at the given value(s) of the independent variable. Simplify the results. (x) = cos 2x (a) (0) (b) (- /4) (c) (/3) (d) ()
-
Draw all significant resonance structures for each of the following compounds: a. b. c. d. e. f. g. h. i. j. k. l. . z: N.
-
A sealed flask with a capacity of 1.22 dm 3 contains 4.50 g of carbon dioxide. The flask is so weak that it will burst if the pressure exceeds 9.500 10 5 Pa. At what temperature will the pressure of...
-
A balloon filled with 11.50 L of Ar at 18.7C and one atm rises to a height in the atmosphere where the pressure is 207 Torr and the temperature is 32.4C. What is the final volume of the balloon?...
-
X M HUNTER MONTEVERDE, U X Sircon Platform clearninglab.litmos.com/assessment/question?courseld=10889424&moduleld=16564537&questionId=7220591&LPId=204201 unter. X odf Z Insurance Agent &... care...
-
Company ABC manufactures radios and uses direct labor hours (DLH) as its allocation base. The following information relates to the companys manufacturing overhead data for 2021: Budgeted output...
-
[The following information applies to the questions displayed below.] The December 31, 2021, adjusted trial balance for Fightin' Blue Hens Corporation is presented below. Accounts Debit Credit Cash $...

Study smarter with the SolutionInn App


