Bromine is obtained from brine wells. The process involves treating water containing bromide ion with Cl 2
Question:
Bromine is obtained from brine wells. The process involves treating water containing bromide ion with Cl2 and extracting the Br2 from the solution using an organic solvent. Write a balanced equation for the reaction of Cl2 and Br−. What are the oxidizing and reducing agents in this reaction? Using the table of standard reduction potentials (Appendix M), verify that this is a product-favored reaction at equilibrium.
Data given in Appendix M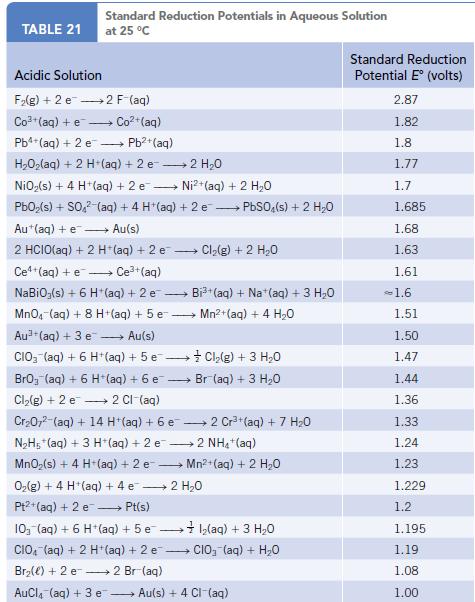
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





