Complete the following nuclear equations. Write the mass number, atomic number, and symbol for the remaining particle.
Question:
Complete the following nuclear equations. Write the mass number, atomic number, and symbol for the remaining particle.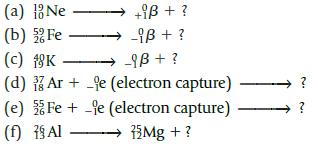
Transcribed Image Text:
(a) Ne (b) Fe +iß + ? -iß + ? (c) 18K > -9B+ ? (d) Ar+ -e (electron capture) - Fee (electron capture) (e) (f) Al 75 Mg + ? 11 IN ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)

Answered By

Akash Goel
I am in the teaching field since 2008 when i was enrolled myself in chartered accountants course
Since then i have an experience of teaching of class XI, XII, BCOM, MCOM, MBA, CA CPT.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Complete the following nuclear equations. Write the mass number, atomic number, and symbol for the remaining particle. (a) (b) Kr 36 Ag (c) 23 Pa (d) 23 Th 90 (e) (f) ? Br 12 111 48 -i + ? 23Ac + ?...
-
Complete the following nuclear equations. Write the mass number, atomic number, and symbol for the remaining particle. (a) Be + ? (b) ? + in (c) Ca + ? (d) 2 Am + He 96 (e) 246Cm + C (f) 23 U + ? Li...
-
Complete the following nuclear equations and identify X in each case: 59 (b) 21 53 200
-
Look at the following code. Which line will cause a compiler error? Explain why. Line 1 public class ClassA Line 2 { Line 3 public ClassA () {} Line 4 public final int methodl (int a) () Line 5...
-
Delaware Chemical Company manufactures specialty chemicals by a series of three processes, all materials being introduced in the Distilling Department. From the Distilling Department, the materials...
-
If a U.S. company exports its goods to Japan, how would it use a futures contract on Japanese yen to hedge its exchange rate risk? Would it buy or sell yen futures? Does the way the exchange rate is...
-
Do you agree with Herzbergs motivational factors? Explain. Has anything been left out of his model?
-
Rick Williams, a systems analyst, and Carla Moore, a programmer/ analyst, completed a set of DFDs representing a data and process model of the SWL payroll system project. Rick had recently attended a...
-
Novak Inc. reports the following pretax income (loss) for both book and tax purposes. Year 2018 2019 2020 2021 N Pretax Income (Loss) $123,000 98,000 (270,000) 110,000 Tax Rate 20% 20% 25% 25% The...
-
The uranium-235 radioactive decay series, beginning with and ending with occurs in the following sequence: , , , , , , , , , , . Write an equation for each step in this series. 2351 92 gu
-
What are the advantages and disadvantages of food preservation using radiation?
-
An organization has been assigned the prefix 200.1.1/24 (a class C) and wants to form subnets for four departments, with hosts as follows: There are 145 hosts in all. (a) Give a possible arrangement...
-
How do different generational cohorts within the workforce perceive and contribute to organizational culture, and what steps can organizations take to create a cohesive culture that bridges...
-
How do various tools and techniques such as adding slack (padding estimates) or project buffers help project managers perform duration estimates? What are some ethical considerations when using slack...
-
How do you assess the managerial challenge posing the decision of having an organization-wide uniform package of compensation and benefits in the present context of organizations having diversity of...
-
what you have to do is make order decisions based on the sales, stock, and delivery cycle of each item. You are making decisions of marking orders from suppliers, and they will deliver the item next...
-
How do advanced relaxation techniques, such as progressive muscle relaxation or guided imagery, contribute to a comprehensive stress management plan ?
-
Determine the product E3 E2 E1 of the elementary matrices in (1.19). Is this the same as the product E1 E2 E3? Which is easier to predict? -1 0 0 I
-
A number of years ago the United Food and Commercial Workers Union organized 800 workers of the 1035 employees at one of the Wilson Brothers food operations in Toronto, Ontario. The employees include...
-
Inner ear. A student constructs a model of the utricle of the ear by attaching wooden balls (m = 0.080 kg) by strings to the bottom of a fish tank and then submerging them in water so that they float...
-
Two workers must slide a crate designed to be pushed with a rigid rod (on the left in Fig. P4.73) and at the same time pulled with a rope (on the right). The crate has mass m = 45 kg, the angle =...
-
A bartender slides a mug of root beer with mass m = 2.6 kg down a bar top of length L = 2.0 m to an inattentive patron who lets the mug fall a height h = 1.1 m to the floor. The bar top (Fig. P4.72)...
-
2/26/17 1/13/17 5/14/17 3/29/18 4/14/18 9/29/18 6/24/17 5/22/18 3/1/17 5/23/17 12/26/17 2/24/18 10/30/17 4/16/17 5/22/17 2/22/18 1/17/17 8/2/18 7/23/17 11/17/17 8/8/18 1/7/17 3/10/18 6/16/17 5/31/18...
-
nel A company has gathered data to be used in preparing the statement of cash flows (indirect method). Listed below, in no particular order, are items to be included in that statement. 50 Purchase of...
-
Mercer Asbestos Removal Company removes potentially toxic asbestos insulation and related products from buildings. There has been a long-simmering dispute between the company's estimator and the work...

Study smarter with the SolutionInn App


