In the presence of oxgyen and acid, two half-reactions responsible for the corrosion of iron are Calculate
Question:
In the presence of oxgyen and acid, two half-reactions responsible for the corrosion of iron are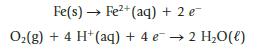
Calculate the the standard potential, E°, and decide whether the reaction is product-favored at equilibrium. Will decreasing the pH make the reaction less thermodynamically product-favored at equilibrium?
Transcribed Image Text:
Fe(s)→ Fe²+ (aq) + 2 e O₂(g) + 4 H+ (aq) + 4 e → 2 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Calculation of the standard potential E The standard potential of a reaction is calculated by subtra...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
In the presence of the enzyme aconitase, the double bond of aconitic acid undergoes hydration. The reaction is reversible, and the following equilibrium is established: (a) The major tricarboxylic...
-
In a study to compare two different corrosion inhibitors, specimens of stainless steel were immersed for four hours in a solution containing sulfuric acid and a corrosion inhibitor. Forty-seven...
-
In the presence of oxygen and water, two half-reactions responsible for the corrosion of iron are Calculate the the standard potential, E, and decide whether the reaction is product-favored at...
-
If the appropriate discount rate for the following cash flows is 7.13 percent per year, what is the present value of the cash flows? Year Cash Flow 1 ......................$1,400 2...
-
Dayton Industrial produces a variety of chemicals that are used in an array of commercial applications. One popular product, a chemical solvent, contains two very caustic acids, A and B, each of...
-
What is adverse selection? What is moral hazard? Give an example of these two problems arising between a firm and its suppliers.
-
Is Mr. Sweeney liable for the outstanding debts of the limited partnership?
-
The comparative consolidated statement of financial position at December 31, Year 2, and the consolidated income statement for Year 2, of Parent Ltd. and its 70%-owned subsidiary are shown below....
-
Xinhong Company is considering replacing one of its manufacturing machines. The machine has a book value of $38,000 and a remaining useful life of four years, at which time its salvage value will be...
-
Balance the following equations. (a) Zn(s) + VO+ (aq) Zn+ (aq) + V+ (aq) (b) Zn(s) + VO3(aq) V2+ (aq) + Zn+ (aq) (c) Zn(s) + CIO- (aq) Zn(OH)(s) + Cl(aq) (d) CIO- (aq) + [Cr(OH)4](aq) Cl(aq) +...
-
Use E values to predict which of the following metals, if coated on nickel, will provide cathodic protection against corrosion to nickel. (a) Cu (b) Mg (c) Zn (d) Cr
-
In Problems 7 14, find the value of each permutation. P(8, 8)
-
3. Now the bomb arrives. Please catch fx,y(x, y) = = cx cx - dy, where 0 < x < 1, 0 y x. 13 a) Please find coefficients c, d such that cd= 8 b) Please find fx(x) and fy (y). Are X and Y independent?...
-
At March 31, account balances after adjustments for Vizzini Cinema are as follows: Account Balances Accounts Cash Supplies Equipment (After Adjustment) $11,000 4,000 50,000 Accumulated...
-
2. "A student holds a thin aluminum pie pan horizontally 2 m above the ground and releases it. Using a motion detector, she obtains the graph shown in Figure P3.12. Based on her measurements, (a)...
-
Mark has two sticks, 25 inches, and 20 inches. If he places them end-to-end perpendicularly, what two acute angles would be formed when he added the hypotenuse?
-
A wedding website states that the average cost of a wedding is $29,205. One concerned bride hopes that the average is less than reported. To see if her hope is correct, she surveys 36 recently...
-
Find vectors x and y in R2 that are orthonormal with respect to the inner product (u, v) = 3u1v1 + 2u2v2 but are not orthonormal with respect to the Euclidean inner product.
-
After looking at the resources, explain what a spirit image is. Why might looking at a god and/or a human in terms of their spirit be helpful if you want to eliminate some of the divisions between...
-
Draw an SM chart for the square root circuit of Problem 4.14. Data from Problem 4.14. This problem involves the design of a circuit that finds the square root of an 8-bit unsigned binary number N...
-
Draw an SM chart for the binary multiplier of Problem 4.22. Data from Problem 4.22. Design a multiplier that will multiply two 16-bit signed binary integers to give a 32-bit product. Negative numbers...
-
Design a binary-to-BCD converter that converts a 10-bit binary number to a 3-digit BCD number. Assume that the binary number is 999. Initially the binary number is placed in register B. When an St...
-
5. Volunteer Manufacturing was acquired by Trajectory Manufacturing. Trajectory was interested in expanding its product line to include Volunteer's most popular Volunteer provided Trajectory with the...
-
1. What is amount of cash inflow from operations? 2. What is amount of cash outflow from operations? 3. What is amount of loan balance at the end of January after loan repayments, if any? XYZ...
-
On January 1, 2020, Palka, Inc., acquired 70 percent of the outstanding shares of Sellinger Company for $1,277,500 in cash. The price paid was proportionate to Sellingers total fair value, although...

Study smarter with the SolutionInn App


