Phenol (C 6 H 5 OH), commonly called carbolic acid, is a weak organic acid. If you
Question:
Phenol (C6H5OH), commonly called carbolic acid, is a weak organic acid.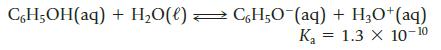
If you dissolve 0.195 g of the acid in enough water to make 125 mL of solution, what is the equilibrium hydronium ion concentration? What is the pH of the solution?
Transcribed Image Text:
CH₂OH(aq)+ H₂O(l) → CH₂O¯(aq) + H₂0+ (aq) K₂ 1.3 X 10-10 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To find the equilibrium hydronium ion concentration and the pH of the solution we can use the inform...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Phenol, C 6 H 5 OH, is a weak organic acid. Suppose 0.515 g of the compound is dissolved in enough water to make 125 mL of solution. The resulting solution is titrated with 0.123 M NaOH. (a) What is...
-
You make a solution by dissolving 0.0010 mol of HCl in enough water to make 1.0 L of solution. a. Write the chemical equation for the reaction of HCl(aq) and water. b. Without performing...
-
Assume you dissolve 0.235 g of the weak acid benzoic acid, C 6 H 5 CO 2 H, in enough water to make 1.00 10 2 mL of solution and then titrate the solution with 0.108 M NaOH. (a) What was the pH of...
-
FIGURE P15.62 is a top view of an object of mass m connected between two stretched rubber bands of length L. The object rests on a frictionless surface. At equilibrium, the tension in each rubber...
-
Under what conditions could it be considered acceptable to hire a relative of an employee or executive in a public organization, such as in federal government, or a publicly owned business, such as...
-
1. How can companies benefit from the cultural assessments regularly performed by Mattel? How could the information obtained be used to create business value for those organizations? Provide multiple...
-
In what way is the automobile no-fault concept similar to workers compensation? In what way is it different?
-
Stratco Corporation computed a pretax financial income of $40,000 for the first year of its operations ended December 31, 2008. Included in financial income was $50,000 of nontaxable revenue, $20,000...
-
Which of the following is an example of a variable consideration? John is expected to receive $100 for his tutoring services provided that he keeps track of his hours Melody's Piano will get paid for...
-
What are the equilibrium concentrations of H 3 O + , CN , and HCN in a 0.025 M solution of HCN? What is the pH of the solution?
-
A 15-L flask at 300 K contains 6.44 g of a mixture of NO 2 and N 2 O 4 in equilibrium. What is the total pressure in the flask? (K p for 2 NO 2 (g) N 2 O 4 (g) is 7.1.)
-
Suppose that the Producer Price Index and the sales of Hoskins Wholesale Distributors for 1995 and 2012 are: What are Hoskins real sales (also called deflated sales) for the 2years? Year 1995 2012...
-
MATH 115 Precalculus 4. Determine the interval(s) on which the function is decreasing. Summer, 2017, V3.1 4. A. (-00, -2.5) and (1,4.5) B. (-0, -1) and (3,) C. (-1,3) D. (-1.3, 1.3) 5. Which of the...
-
Let y= f(x) be a differentiable function such that dy = and f(8) 2. What is the approximation of f(8.1) using the line dx tangent to the graph of at a = 8?
-
Let f(x)=.If the rate of change of f at x=c is twice its rate of change at x=1, then c = ( )
-
What is the benefit of reviewing the companys balace sheet? What activities has the company undertaken in 2014 and 2015 figures? How can you tell if the company is doing well? What should the company...
-
1. Describe the challenges Lucky Air faced when collecting fares directly from customers. 2. How did Lucky Air usually sell tickets? 3. Identify the four functions of money. 4. Which of the four...
-
A right circular cylinder of radius r is inscribed in a sphere of radius 2r. Find a formula for V(r), the volume of the cylinder, in terms of r.
-
Frontland Advertising creates, plans, and handles advertising campaigns in a three-state area. Recently, Frontland had to replace an inexperienced office worker in charge of bookkeeping because of...
-
Solve the equations in Prob. A18 using the Gauss elimination method. Data From Prob. A-18 4x 1 + x 2 + x 3 = -1, -5x 1 + 4x 2 + 3x 3 = 4, x 1 - 2x 2 + x 3 = 2 Using the matrix equation x = A -1 C.
-
Solve the equations x 1 + 2x 2 - 2x 3 = 5, x 1 - x 2 + x 3 = -1, x 1 - x 2 - x 3 = 1 Using the matrix equation x = A -1 C.
-
Solve the equations in Prob. A20 using the Gauss elimination method. Data From Prob. A-20 x 1 + 2x 2 - 2x 3 = 5, x 1 - x 2 + x 3 = -1, x 1 - x 2 - x 3 = 1 using the matrix equation x = A -1 C.
-
On January 1, 2020, Riverbed Limited paid $529,836.45 for 10% bonds with a maturity value of $510,000. The bonds provide the bondholders with a 9% yield. They are dated January 1, 2020, and mature on...
-
please answer 2&3. Thanks! 2. The following information was taken from the financial records for Whitlock Industries for the years 2019 and 2020. The balance sheet items were recorded at the end of...
-
Alex and Bess have been in partnership for many years. The partners, who share profits and losses on a 60:40 basis, respectively, wish to retire and have agreed to liquidate the business. Liquidation...

Study smarter with the SolutionInn App


