The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed
Question:
The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed by measuring the appearance of p-nitrophenol in the reaction mixture: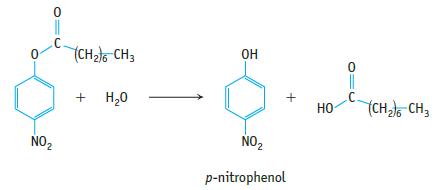
The following data were obtained at 30°C: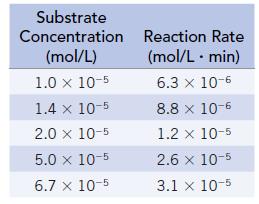
This reaction obeys Michaelis-Menten kinetics (Section 14.6). Determine the value of Ratemax for this reaction using the method described in Study Question 14.45.
Data given in Question 14.45
According to the Michaelis–Menten model, if 1/ Rate is plotted versus 1/[S], the intercept of the plot (when 1/[S] = 0) is 1/Ratemax. Using the data below at a given temperature, for a given enzyme and its substrate (S), calculate the maximum rate of the reaction, Ratemax.![[S], mol/L 2.500 1.00 0.714 0.526 0.250 Rate, mmol/min 0.588 0.500 0.417 0.370 0.256](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/2/2/0/9286538cb805e6d31698220928501.jpg)
Data given in Section14.6
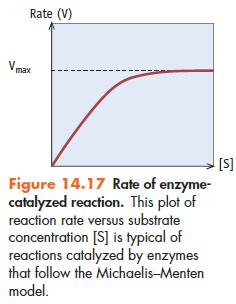
At low substrate concentrations, the first-order kinetics indicates that adding more substrate will increase the rate of the reaction. Why then do the kinetics change at high substrate concentration? Because there is only so much enzyme present, the active sites in the available enzyme molecules become saturated at high substrate concentrations, and the rate reaches its maximum value. Michaelis and Menten were further able to show that their mechanism led to the following equation for the rate (or velocity) of the reaction, V.![V || Vmax[S] KM + [S]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/2/2/1/5736538ce0586a5b1698221573653.jpg)
where Vmax is the maximum rate of the reaction and KM is a constant called the Michaelis constant. When V is plotted versus [S], this equation fits the curve in Figure 14.17 well.
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





