The halogens form three stable, weak acids, HOX. (a) Which is the strongest of these acids? (b)
Question:
The halogens form three stable, weak acids, HOX.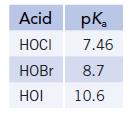
(a) Which is the strongest of these acids?
(b) Explain why the acid strength changes as the halogen atom is changed.
Transcribed Image Text:
Acid pK₂ HOCI 7.46 HOBr 8.7 HOI 10.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 28% (7 reviews)
a Strongest Acid HOCl Hypochlorous Acid The strength of an acid is determined by its acid dissociati...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
The following information was extracted from Citigroup, Inc.'s 2009 annual report. From letter to shareholders: Financial Strength While Citi started the year as a TARP institution receiving...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
A database is to be made to store information about a catalogue of CDs. Information to be stored about each CD includes title, price, genre, and a list of tracks. Each CD will also have an artist,...
-
1. Do you think that TI took the right approach to achieving better customer satisfaction by training its executives first? Would TI have achieved quicker results by training its front-line employees...
-
In the audit of the Regional Transport Company, a large branch that maintains its own bank account, cash is periodically transferred to the central account in Cedar Rapids. On the branch account's...
-
Consider the following hypothesis test: A sample of 50 provided a sample mean of 14.15. The population standard deviation is 3. a. Compute the value of the test statistic. b. What is the p-value? c....
-
1. The University of Pittsburgh Medical Center (UPMC) relies on information systems to operate 19 hospitals, a network of other care sites, and international and commercial ventures. Demand for...
-
Ch . 1 9 Lab Activity Print Item product ine. \ table [ [ , \ table [ [ Thousand ] , [ Board Feet ] ] , \ table [ [ Percentage of ] , [ Board Feet ] ] , , \ table [ [ By - product ] , [ Revenue ] ] ,...
-
Perchloric acid behaves as an acid, even when it is dissolved in sulfuric acid. (a) Write a balanced equation showing how perchloric acid can transfer a proton to sulfuric acid. (b) Draw a Lewis...
-
The acidity of the oxoacids was described in Section 16.9, and a larger number of acids are listed in the table below. (a) What general trends do you see in these data? (b) What has a greater effect...
-
What is the result corresponding to that given in Problem 31.7, for the CIR model. Use maximum likelhood methods to estimate the a, b, and parameters for the CIR model using the same data as that...
-
When do we use Fourier transforms and Laplace transforms in RC/RL/RLC circuit analysis?
-
What are the protocols used in a drone?
-
How do we design a drone?
-
What are the different types of drones?
-
What are the applications of drones?
-
Let the piecewise smooth, simple closed curve C be the boundary of a region S in the xy-plane. Modify the argument in Example 2 to show that (-y) dx A(S)
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
What is the definition of zero energy employed in constructing the statistical mechanical expression for the equilibrium constant? Why was this definition necessary?
-
Why should the equilibrium constant be dependent on the difference in Gibbs energy? How is this relationships described using statistical mechanics?
-
For the equilibrium involving the dissociation of a diatomic, what energetic degrees of freedom were considered for the diatomic and for the atomic constituents?
-
08: When furniture value increased from OMR 10.000 to OMR 12.000, then revaluation account is c. Debited with OMR 2,000 a. Debited with OMR 12,000 d. Credited with OMR 2.000 b. Credited with OMR...
-
Data Table 1 Month January February March April May June July August September October November December Totals Labor Hours 1,262 962 1,318 1,280 942 1,188 1,114 1,006 1,344 1,196 760 792 13,164...
-
Cullumber Tool Company's December 3 1 year - end financial statements contained the following errors. \ table [ [ , December 3 1 , 2 0 2 5 , December 3 1 , 2 0 2 6 ] , [ Ending inventory,$ 8 , 8 0 0...

Study smarter with the SolutionInn App


