The presence of arsenic in a sample that may also contain another Group 5A element, antimony, can
Question:
The presence of arsenic in a sample that may also contain another Group 5A element, antimony, can be confirmed by first precipitating the As3+ and Sb3+ ions as yellow solid As2S3 and orange solid Sb2S3. If aqueous HCl is then added, only Sb2S3 dissolves, leaving behind solid As2S3. The As2S3 can then be dissolved using aqueous HNO3.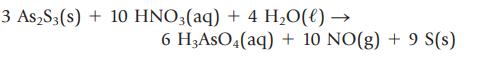
Finally, the presence of arsenic is confirmed by adding AgNO3 to the solution of H3AsO4 to precipitate a reddish brown solid AgxAsOy. The composition of this solid is As, 16.199% and Ag, 69.964%.
(a) What are the oxidation numbers of As, S, and N in the reaction of As2S3 with nitric acid?
(b) What is the formula of the reddish brown solid AgxAsOy?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





