The Tollens test for the presence of reducing sugars (say, in a urine sample) involves treating the
Question:
The Tollen’s test for the presence of reducing sugars (say, in a urine sample) involves treating the sample with silver ions in aqueous ammonia. The result is the formation of a silver mirror within the reaction vessel if a reducing sugar is present. Using glucose, C6H12O6, to illustrate this test, the oxidation–reduction reaction occurring is
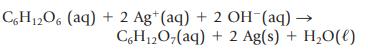 What has been oxidized, and what has been reduced? What is the oxidizing agent, and what is the reducing agent?
What has been oxidized, and what has been reduced? What is the oxidizing agent, and what is the reducing agent?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





