Uracil is a base found in RNA. Indicate sites in the molecule where hydrogen bonding is possible
Question:
Uracil is a base found in RNA. Indicate sites in the molecule where hydrogen bonding is possible or that are sites of Lewis basicity.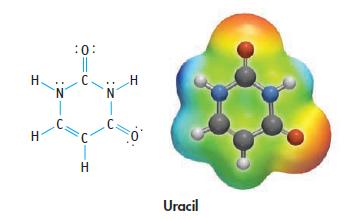
Transcribed Image Text:
H H N :0: || C N 1 C. H Uracil
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Uracil has two main functional groups that are relevant to hydrogen bonding and Lewis basic...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Proteins are synthesized with a particular amino acid sequence through the translation of information encoded in messenger RNA by an RNAprotein complex called a ribosome. Amino acids are specified by...
-
What is the value of y[1] [2] ? int y[3][3]={{1,2,3},{4,5,6}, {7,8,9}};
-
The RNA base uracil is a pyrimidine derivative, usually depicted in its keto form (page 396). The enol tautomer readily undergoes electrophilic substitution, unlike most pyrimidines. a. Draw the...
-
The operations manager of a company that produces frozen dinners had received numerous complaints from supermarkets about the companys Chick-n-Gravy dinners. The manager spilled/mixed items,...
-
Indicate what training methods you would use for each of the following jobs. Give reasons for your choices. a. File clerk b. Computer operator c. Automobile service station attendant d. Pizza maker...
-
Explain the relationships among the initial assessed control risk, tests of controls and substantive tests of transactions for cash receipts, and the tests of details of cash balances.
-
Consider the following hypothesis test: A sample of 75 is used and the population standard deviation is 10. Compute the p-value and state your conclusion for each of the following sample results. Use...
-
Sanders Co. is planning to finance an expansion of its operations by borrowing $150,000. City Bank has agreed to loan Sanders the funds. Sanders have two repayment options: (1) To issue a note with...
-
On January 2, 2015, Bramble Corporation issued $2,000,000 of 10% bonds at 99 due December 31, 2024. Interest on the bonds is payable annually each December 31. The discount on the bonds is also being...
-
Chemists often refer to the degree of ionization of a weak acid or base and give it the symbol . The equilibrium constant in terms of and Co, the initial acid or base concentration, is given by the...
-
You purchase a bottle of water. On checking its pH, you find that it is not neutral, as you might have expected. Instead, it is slightly acidic. Why?
-
Appendix A presents PepsiCos financial statements. Appendix B presents Coca-Colas financial statements. Instructions (a) Based on the information contained in these financial statements, determine...
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob O'Donnell, a local merchant, to contribute the capital to form a partnership....
-
Exercise 6-10A (Algo) Double-declining-balance and units-of-production depreciation: gain or loss on disposal LO 6-3, 6-4, 6-5 Exact Photo Service purchased a new color printer at the beginning of...
-
Independent Events Again assume that when randomly selecting a speaking character in a movie, the probability of getting a female is 0.331, as in Exercise 1. If we want to find the probability of 20...
-
As an official sponsor of the Olympics, what specific benefit did John Hancock use to help drive sales in their national offices?
-
assumes that Nia has both a discount rate of zero and faces an interest rate of zero. These assumptions made calculating her constant level of consumption expenditure of $56,000 fairly...
-
Area of a Polygon Let V0(x0, y0), V1(x1, y1),....., Vn(xn, yn), be the vertices of a simple polygon P, labeled counter clock wise and with V0 = Vn. Show each of the following? (a) (c x dy = 1 / 2 (x1...
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
The constant volume heat capacity for all monatomic gases is 12.48 J mol 1 K 1 . Why?
-
When are rotational degrees of freedom expected to contribute R or 3/2 R (linear and nonlinear, respectively) to the molar constant volume heat capacity? When will a vibrational degree of freedom...
-
The molar constant volume heat capacity of N 2 is 20.8 J mol 1 K 1 . What is this value in terms of R? Can you make sense of this value?
-
On March 11, at the end of a pay period. Global Filter Corp's Payroll Register showed that its 23 empkypes had earned $21.000 of salos salaries and 54 root office salaries Withholdings from the...
-
3. Special Orders (1.5pts): Dog Man Donuts, Inc. makes and sells pre-packaged donuts. Each pre-packaged box of donuts regularly sells for $8.00 each. The firm typically produces and sells 1,600 boxes...
-
The bookkeeper for Nash Company has prepared the following balance sheet as of July 31, 2020. Cash Accounts receivable (net) Inventory Equipment (net) Patents NASH COMPANY BALANCE SHEET AS OF JULY...

Study smarter with the SolutionInn App


