Use K sp values to decide which compound in each of the following pairs is more soluble.
Question:
Use Ksp values to decide which compound in each of the following pairs is more soluble. (Appendix J.)
(a) AgBr or AgSCN
(b) SrCO3 or SrSO4
(c) AgI or PbI2
(d) MgF2 or CaF2
Data given in Appendix J

Transcribed Image Text:
TABLE 18A Solubility Product Constants at 25 °C Cation Compound Cation Ba²+ *BaCrO Hg₂²+ 2+ BaCO3 BaF₂ *BaSO4 Ca²+ Cut, Cu²+ Au+ Fe²+ Pb²+ Mg²+ Mn²+ CaCO3 (calcite) *CaF2 *Ca(OH)2 CaSO4 CuBr Cul Cu(OH)2 CuSCN AuCl FeCO3 Fe(OH)2 PbBr₂ PbCO3 PbCl₂ PbCrO₁ PbF2 Pbl₂ Pb(OH)2 PbSO4 MgCO3 MgF2 Mg(OH)2 MnCO3 *Mn(OH)2 Ksp 1.2 x 10-10 2.6 × 10-9 1.8 x 10-7 1.1 x 10-10 3.4 x 10-9 5.3 x 10-11 5.5 x 10-5 4.9 x 10-5 6.3 x 10-9 1.3 x 10-12 2.2 x 10-20 1.8 x 10-13 2.0 x 10-13 3.1 x 10-11 4.9 × 10-17 6.6 x 10-6 7.4 x 10-14 1.7 x 10-5 2.8 x 10-13 -13 3.3 x 10-8 9.8 x 10-9 1.4 x 10-15 2.5 x 10-8 6.8 x 10-6 5.2 x 10-11 5.6 x 10-12 2.3 × 10-11 1.9 x 10-13 Ni²+ Ag+ Sr²+ TI+ Zn²+ Compound *Hg₂Br₂ Hg₂Cl₂ *Hg2l2 Hg2SO4 NICO3 Ni(OH)2 *AgBr *AgBrO3 AgCH3CO₂ AgCN Ag₂CO3 *Ag₂C₂04 *AgCl Ag₂ CrO4 *Agl AgSCN *Ag₂SO4 SrCO3 SrF₂ SrSO4 TIBr TICI TII Zn(OH)₂ Zn(CN)₂ Ksp 6.4 x 10-23 1.4 x 10-18 2.9 x 10-29 6.5 x 10-7 1.4 x 10-7 5.5 x 10-16 5.4 x 10-13 5.4 x 10-5 1.9 x 10-3 6.0 x 10-17 8.5 x 10-12 5.4 x 10-12 1.8 x 10-10 1.1 x 10-12 8.5 x 10-17 1.0 x 10-12 1.2 x 10-5 5.6 x 10-10 4.3 × 10-9 3.4 x 10-7 3.7 x 10-6 1.9 x 10-4 5.5 x 10-8 3 x 10-17 8.0 x 10-12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
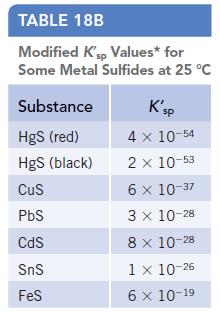
Use K sp values to decide which compound in each of the following pairs is more soluble. (Appendix J.) (a) PbCl 2 or PbBr 2 (b) HgS or FeS (c) Fe(OH) 2 or Zn(OH) 2 Data given in Appendix J TABLE 18A...
-
Which compound in each of the following pairs of compounds is the more soluble one? a. Silver chloride or silver iodide b. Magnesium hydroxide or copper(II) hydroxide
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
Table P-23 contains Southwest Airlines' quarterly income before extraordinary items ($MM) for the years 1988-1999. a. Plot the income data as a time series and describe any patterns that exist. b. Is...
-
1. Given the facts of this case, should John have been discharged? Explain. 2. Should the sales representatives of AEM be held to a higher standard of personal conduct than sales representatives for...
-
The minutes of the board of directors of the Meeting of March 5, 2011 The meeting of the board of directors of Tetonic Metals was called to order by the James Cook, chairman of the board, at 8:30 am....
-
Consider a regression study involving a dependent variable y, a categorical independent variable x1, and a categorical variable with two levels (level 1 and level 2). a. Write a multiple regression...
-
Steamboat Co. expects to maintain the same inventories at the end of 2010 as at the beginning of the year. The total of all production costs for the year is therefore assumed to be equal to the cost...
-
Smart Stream Inc. uses the variable cost method of applying the cost - plus approach to product pricing. The costs of producing and selling 1 0 , 0 0 0 cell phones are as follows: Variable costs per...
-
Calculate the molar solubility of silver thiocyanate, AgSCN, in pure water and in water containing 0.010 M NaSCN.
-
If 55 mg of lead(II) sulfate is placed in 250 mL of pure water, does all of it dissolve? If not, how much dissolves?
-
Explain why delta hedging is easier for Asian options than for regular options.
-
time complexity of the following algorithm forn-1 to n-1 do for je +1 to n do Print & for Kn-3 to n+4 do print k
-
There are various compounds and epoxies that have been used to "final bed" rifle stocks. In your opinion, which is the best and why? Does this depend on the material of the stock? If so, why? Conduct...
-
Taylor Series: Problem 3 Previous Problem Problem List Next Problem 8 (5 points) Write the Taylor series for f(x) = x about x = 2 as C, (x 2)". Find the first five coefficients. n=0 - Co= C1= C2= C3=...
-
Using the figure below, draw the FBD , ?Shear Force and Bending Moment diagrams and find the maximum internal moment for the beam shown. 10 kNm 10 kN 3 m
-
Suppose the goods market is: Y = 1800 - 100i and the LM curve Y = 500 +591, where x is the last digit of your ID number. Determine the equilibrium income (Y), interest rate (i). Explain the role of...
-
Solve y" - 2y' + 2y = 0 and express your answer in the form ceax sin (βx + (). Let sin ( = C1 / c and cos ( = C2 / c, where VC} + C3.
-
(8%) Problem 6: A student attaches a f= 3.5 kHz oscillator to one end of a metal rail of length L = 25 m. The student turns on the oscillator and uses a piezoelectric gauge at the other end to...
-
Explain why two magnetic fields, a static field and a radiofrequency field, are needed to carry out NMR experiments. Why must the two field directions be perpendicular?
-
Explain the difference in the mechanism that gives rise to through-space dipoledipole coupling and through-bond coupling.
-
Predict the number of chemically shifted + 1H peaks and the multiplet splitting of each peak that you would observe for diethyl ether. Justify your answer.
-
Parker Plastic, Inc., manufactures plastic mats to use with rolling office chairs. Its standard cost Information for last year follows: Standard Standard Price Quantity (Rate 10 sq ft. $ 1.13 per sq....
-
A company would like to borrow a $1 million loan from the bank for 4 years. This borrower has a BB credit rating on the bond market. The bank has collected related corporate bond returns and...
-
Orlando Investments is buying an office building in Orlando, FL. - Building's asking price: $2,000,000. - The company is planning to accept the asking price and take a loan with an LTV of 72%. - The...

Study smarter with the SolutionInn App


