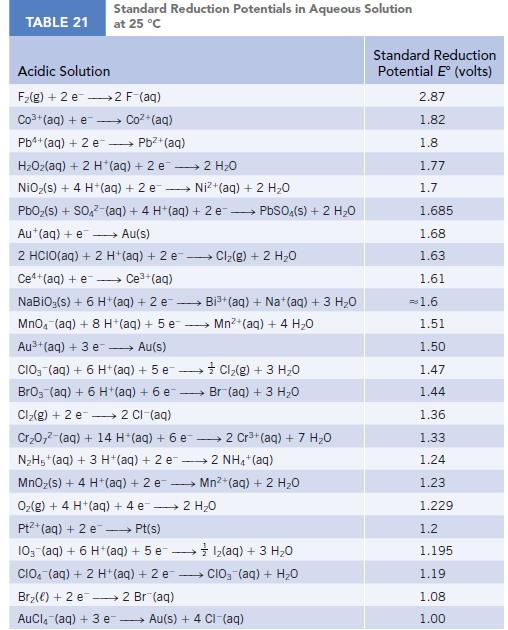
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following
Question:
Use the table of standard reduction potentials (Appendix M) to calculate ΔrG° for the following reactions at 298 K.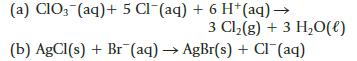
Data given in Appendix M
Transcribed Image Text:
(a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) → 3 Cl₂(g) + 3 H₂O(l) (b) AgCl(s) + Br (aq) → AgBr(s) + Cl(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a The relation between Gibbs free energy and ...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) 3 Cu(s) + 2NO3(aq) + 8 H+ (aq) 3 Cu+ (aq) + 2 NO(g) +...
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Fe(s) + Ni2+(aq) Fe2+ (aq) + Ni(s) (b) Co(s) + 2 H+...
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Cu(s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag(s) (b) 3 Ce4+...
-
Built-Tite uses job order costing. The T-account below summarizes Factory overhead activity for the current year. Factory Overhead Debit Credit 16,200 106,600 25,200 60,200 1. Compute total applied...
-
Southern Rail Inc. is considering acquiring equipment at a cost of $442,500. The equipment has an estimated life of 10 years and no residual value. It is expected to provide yearly net cash flows of...
-
Shown are partial financial statements for Garners' Platoon Mental Health Care, Inc. Fill in the blanks on the four financial statements. Garners Platoon Mental Health Care, Inc. Balance Sheet as of...
-
Why do some employees of an organization behave unethically? Why is it necessary for an organization to develop employees ethics? AppendixLO1
-
The Clarkson Company recently reported net profits after taxes of $15.8 million. It has 2.5 mil-lion shares of common stock outstanding and pays preferred dividends of $1 million a year. The companys...
-
$1.08 Constellation Brands, a U.S. company, purchases merchandise from a German supplier on a regular basis. On April 1, 2020, Constellation purchased 100,000 for delivery on June 30, 2020, in...
-
In 2005, global SO 2 emission was estimated to be 12.83 Gg (gigagrams). According to the EPA, 71% of SO 2 emissions into the atmosphere is from coalfired power plants. How much coal (in metric tons)...
-
Calculate equilibrium constants for the following reactions at 298 K. Indicate whether the equilibrium as written is reactant- or product-favored at equilibrium. (a) 2 Cl(aq) + Br(e) (b) Fe+ (aq) +...
-
Explain the term management accounting and state what you understand to be its main objectives.
-
Suppose that f(x) = 8x + 5. (A) Find the slope of the line tangent to f(x) at x = 7. (B) Find the instantaneous rate of change of f(x) at x = -7. C) Find the equation of the line tangent to f(x) at x...
-
Whichof the following regarding the relationship between business risk and financial risk is least accurate based on our discussions in class? A. Business risk represents uncertainty caused by...
-
2. Question 2 When preparing a financial spread analysis, what should be done when the financial statement captions don't align with those provided in the spread template? 1 point Conform the...
-
Your company just secured an $6 million contract with a major public-sector client that is expected to generate thousands of jobs over the next 10 years. Describe the scenario as a blog.
-
Dr. John Gottman's research has been able to accurately predict divorce more than 90% of the time.By carefully studying how couples interact with each other, he identified what are known as "The Four...
-
Suppose that the characteristic polynomial of some matrix, 4 is found to be p() = ( - 1)( - 3)2(-4)3 In each part, answer the question and explain your reasoning (a) What is the size o f A? (b) Is A...
-
Don Griffin worked as an accountant at a local accounting firm for five years after graduating from university. Recently, he opened his own accounting practice, which he operates as a corporation....
-
Write a Verilog description of the following combinational circuit using concurrent statements. Each gate has a 5-ns delay, excluding the inverter, which has a 2-ns delay. C - ABCDAR
-
(a) Write Verilog code for a full subtracter using logic equations. (b) Write Verilog code for a 4-bit subtracter using the module defined in (a) as a component.
-
Write Verilog code for the following circuit. Assume that the gate delays arenegligible. (a) Using concurrent statements. (b) Using an always block with sequential statements. No latches should be...
-
Cox Automotive in Terre Haute, IN has a margin of safety of $90,000. True or False: If the automotive company's sales drop by $80,000 they will still have positive net operating income
-
Which financial statement shows dividends? O Balance Sheet Income Statement Statement of Retained Earnings Trial Balance
-
Choose a company and explain an example of how customers use this companys resources, services, or programs very differently? How does this impact the companys managerial decision-making?

Study smarter with the SolutionInn App


