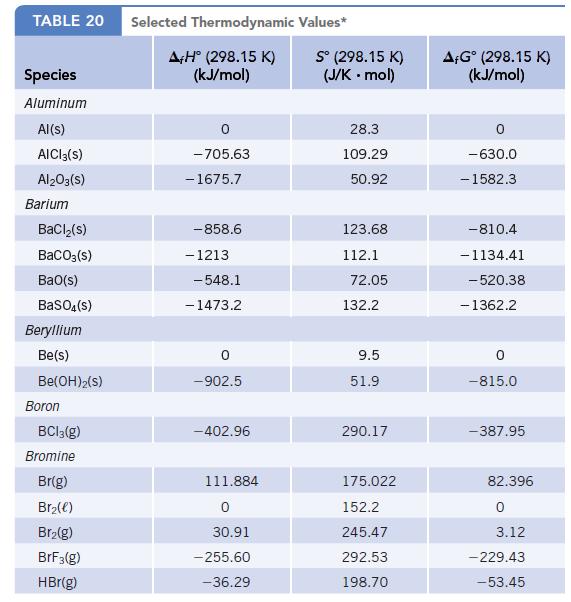
Use values of f G for solid and gaseous iodine at 25C (Appendix L) to calculate
Question:
Use values of Δf G° for solid and gaseous iodine at 25°C (Appendix L) to calculate the equilibrium vapor pressure of iodine at this temperature.
Data given in Appendix L
Transcribed Image Text:
ⒸCengage Learning/Chades D. Winters
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
120 128 AG for 12 000 KJmol AG for I 193 KJmol AG 1x0 193KJmol000 ...View the full answer

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
dC The concentration of a solute at time t is given by C(t) where = 2(20 - C(t)) for t20. dt (a) Solve the differential equation when C(0) = 7. (b) Find limC(t). (c) Use the answer to part (a) to...
-
Use data from Appendix C to calculate the equilibrium constant, K, at 298 K for each of the following reactions: H2(g) + 12(g) 2 HI(g) C2H5OH (g)- C2H4(g) + H2O(g)
-
The following data are the equilibrium vapor pressure of limonene, C 10 H 16 , at various temperatures. (Limonene is used as a scent in commercial products.) (a) Plot these data as ln P versus 1/T so...
-
What does a SWOT analysis reveal about the overall attractiveness of lululemon's situation?
-
In what ways are team cohesiveness and team conflict related?
-
As a muscle car aficionado, a friend of yours likes to restore cars of the 60s and 70s and sell them for a profit. He started his latest project (a 1965 Shelby GT350) four months ago and has a total...
-
Which statement is least appropriate? The CAE should adopt a suitable policy on responses from the client and they may be: a. incorporated into the report. Here adjustment is made throughout the...
-
The trial balance for Pioneer Advertising is shown in Illustration. Instead of the adjusting entries shown in the textbook at October 31, assume the following adjustment data. 1. Supplies on hand at...
-
I'm not sure what would be Bal. ? I messed my chart all up. Not really sure what to do. Homework: Week 2 Homework Assignment Score: 1) 3 of 1 pt HW Score: 32 45%, 1304 pts ustion Help P2-29A (similar...
-
The formation of diamond from graphite is a process of considerable importance. (a) Using data in Appendix L, calculate r S, r H, and r G for this process at 25C. (b) The calculations will suggest...
-
Oxygen dissolved in water can cause corrosion in hot-water heating systems. To remove oxygen, hydrazine (N 2 H 4 ) is often added. Hydrazine reacts with dissolved O 2 to form water and N 2 . (a)...
-
Three-way catalytic converters have been installed in new vehicles in order to reduce pollutants from motor vehicle exhaust emissions. However, these converters unintentionally increase the level of...
-
Do you support the policy of not allowing some Chinese nationals to attend graduate school in the United States because of national security concerns?
-
Using your product or service name or category, do a search using the following phrase: Find a (insert the name of your product or service here...) near me. For instance, using my Mobile Notary...
-
Why do you think it is important to consider only relevant costs when conducting a differential analysis for a major purchase? Why not consider all possible costs in your decision? provide specific...
-
How do power dynamics and influence tactics shape decision-making processes and organizational politics within hierarchical structures ?
-
How do I answer these given the information below? Loan Amount? Loan to Value? Loan to Cost? Payment amount? Loan Balance at Maturity? Given Information: Property Cost: $1,000,000 Bank Policy on LTV:...
-
Oil is leaking at the rate of V' (t) = 1 - t/110 from a storage tank that is initially full of 55 gallons. How much leaks out during the first hour? During the tenth hour how long until the entire...
-
Presented below are income statements prepared on a LIFO and FIFO basis for Kenseth Company, which started operations on January 1, 2024. The company presently uses the LIFO method of pricing its...
-
Determine the moments at D and C, then draw the moment diagram for each member of the frame. Assume the supports at A and B are pins. EI is constant. 16 kN 3 m D 4 m
-
Determine the moments at B and C, then draw the moment diagram for each member of the frame. Assume the supports at A,E, and D are fixed.EI is constant. 10 k 2 k/ft 8 ft -8 ft PA 12 ft 16 ft
-
The frame is made from pipe that is fixed connected. If it supports the loading shown, determine the moments developed at each of the joints. EI is constant. 18 kN 18 kN 4 m 4 m 4 m
-
M and M, Inc. produces a product that has a variable cost of $3.10 per unit. The company's fixed costs are $43,500. The product is sold for $6 per unit and the company desires to earn a target profit...
-
Schoeck, CPA, is considering leaving a position at a major public accounting firm to join the staff of local bookkeeping and advisory firm that does write-up work, tax preparation and planning, and...
-
On January 1, 2012, Franel Co. acquired all of the common stock of Hurlem Corp. For 2012, Hurlem earned net income of $360,000 and paid dividends of $190,000. Amortization of the patent allocation...

Study smarter with the SolutionInn App


