You and your lab partner are asked to determine the density of an aluminum bar. The mass
Question:
You and your lab partner are asked to determine the density of an aluminum bar. The mass is known accurately (to four significant figures). You use a simple metric ruler to measure its dimensions and obtain the results for Method A. Your partner uses a precision micrometer and obtains the results for Method B. 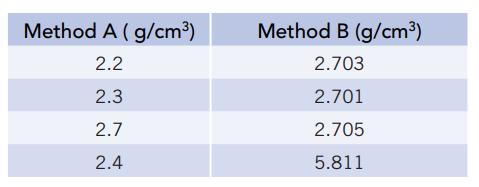
The accepted density of aluminum is 2.702 g/cm3.
(a). Calculate the average density for each method. Should all the experimental results be included in your calculations? If not, justify any omissions.
(b). Calculate the percent error for each method’s average value.
(c). Calculate the standard deviation for each set of data.
(d). Which method’s average value is more precise? Which method is more accurate?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





