You can dissolve an aluminum soft drink can in an aqueous base such as potassium hydroxide. If
Question:
You can dissolve an aluminum soft drink can in an aqueous base such as potassium hydroxide.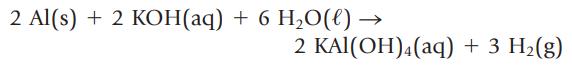
If you place 2.05 g of aluminum in a beaker with 185 mL of 1.35 M KOH, will any aluminum remain? What mass of KAl(OH)4 is produced?
Transcribed Image Text:
2 Al(s) + 2 KOH(aq) + 6 H₂O(l) → 2 KAl(OH)4 (aq) + 3 H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine whether any aluminum remains and to calculate the mass of KAlOH4 produced we can use st...View the full answer

Answered By

Gabriela Rosalía Castro
I have worked with very different types of students, from little kids to bussines men and women. I have thaught at universities, schools, but mostly in private sessions for specialized purpuses. Sometimes I tutored kids that needed help with their classes at school, some others were high school or college students that needed to prepare for an exam to study abroud. Currently I'm teaching bussiness English for people in bussiness positions that want to improve their skills, and preparing and ex-student to pass a standarized test to study in the UK.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
1. What segment of the external environment has more impact on an organization? 2. If an organization wants to engage in a new industry, which area of the industry environment should be the most...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Given that log (2) 0.91 and log (5) 2.1, evaluate each of the following. Hint: use the properties of logarithms to rewrite the given logarithm in terms of the the logarithms of 2 and 5. a) log(0.4)~...
-
Explain what is meant by process capability. Why is it important? What does it tell us? How can it be measured?
-
Solve Problem 4.7 when = 40 o . P4.7 A hand truck is used to move two barrels, each weighing 80 lb. Neglecting the weight of the hand truck, determine (a) The vertical force P which should be...
-
Given that z is a standard normal random variable, find z for each situation. a. The area to the right of z is .01. b. The area to the right of z is .025. c. The area to the right of z is .05. d. The...
-
On December 21, 1988, Pan Am Flight 103 exploded 31,000 feet in the air over Lockerbie, Scotland, killing all 259 passengers and crew on board and 11 people on the ground. Among those killed was...
-
National Supply's shareholders' equity included the following accounts at December 31, 2020: Shareholders' Equity Common stock, 7 million shares at $1 par Pald-in capital-excess of par Retained...
-
What volume of 0.750 M Pb(NO 3 ) 2 , in milliliters, is required to react completely with 1.00 L of 2.25 M NaCl solution? The balanced equation is Pb(NO3)2(aq) + 2 NaCl(aq) PbCl(s) + 2 NaNO3(aq)
-
In the photographic developing process, silver bromide is dissolved by adding sodium thiosulfate. If you want to dissolve 0.225 g of AgBr, what volume of 0.0138 M Na 2 S 2 O 3 , in milliliters,...
-
Find parametric equations for the tangent line to the curve with the given parametric equations at the specified point. x = ln(t + 1), y = t cos 2 t , z = 2 t ; (0, 0, 1)
-
A production Edgeworth Box, with origins indicated for the inputs of capital, K , and labor, L , into production of goods X and Y .Eight isoquants are shown, reflecting standard...
-
For 2014, Nichols, Inc., had sales of 150,000 units and production of 200,000 units. Other information for the year included: Direct manufacturing labor 187,500 Variable manufacturing overhead...
-
reading the following statement and decide whether you agree or disagree with the statement: "The free market system is the best economic system since it is the most efficient and solves basic...
-
find the net presbf value of the project ? present value index? Net present value A project has estimated annual net cash flows of $11,250 for 10 years and is estimated to cost $42,500. Assume a...
-
Calculate the ICER for the new treatment, without adjusting for the health utility index. Assuming the $50K benchmark*, as a clinical decision maker or health policy advisor, would you recommend...
-
Compute the missing amount (?) for each company-amounts in millions. Which company has the Highest net income? Highest percent of net income to revenues? Diamond Corp. Lance Co. (In Millions) $30...
-
6. (Potential Energy and Conservation of Energy) What should be the spring constant k of a spring designed to bring a 1200-kg car to rest from a speed of 95 km/h so that the occupants undergo a...
-
2.25 moles of an ideal gas with C V ,m = 3/2 R undergoes the transformations described in the following list from an initial state described by T = 310.K. and P = 1.00 bar. Calculate q, w, U, H, and...
-
Compounds A, B, C, and D are constitutionally isomeric, aromatic compounds with molecular formula C 8 H 10 . Deduce the structure of compound D using the following clues. The 1 H NMR spectrum of...
-
Consider the reversible Carnot cycle shown in Figure 5.2 with 1.25 mol of an ideal gas with C V = 5/2R as the working substance. The initial isothermal expansion occurs at the hot reservoir...
-
Question 12 (3 points) What must the fraud investigator avoid in his report? Any inferences of guilt. Any financial data for the company. The methods of proving fraud. All of the above. Question 13...
-
QS 23-16 (Algo) Pricing using total cost LO P6 Garcia Company sells snowboards. Each snowboard requires direct materials of $106, direct labor of $36, variable overhead of $51, and variable selling...
-
Steelers Football, Inc. (SFI) needs to prepare a bank reconciliation for September. The information from SFIs bank statement and cash account is summarized below. Bank Statement Cash Account Records...

Study smarter with the SolutionInn App


