A 0.2500-g sample of an AlZn alloy reacts with HCl to form hydrogen gas: The hydrogen produced
Question:
A 0.2500-g sample of an Al–Zn alloy reacts with HCl to form hydrogen gas:
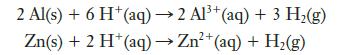
The hydrogen produced has a volume of 0.147 L at 25°C and 755 mm Hg. What is the percentage of zinc in the alloy?
Transcribed Image Text:
2 Al(s) + 6 H+ (aq) → 2 Al³+ (aq) + 3 H₂(g) Zn(s) + 2 H+ (aq) → Zn²+ (aq) + H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
A 6.11-g sample of a Cu-Zn alloy reacts with HCl acid to produce hydrogen gas. If the hydrogen gas has a volume of 1.26 L at 22C and 728 mmHg, what is the percent of Zn in the alloy? (Cu does not...
-
Rank the following alkenes in order of their stability, Put the most stable first I II III IV O A. IV > || > II>| O B. IV > || >|> II OC. II > I| >1> IV O D.I> I| > III > IV
-
(A) The reaction of aluminum with hydrochloric acid produces hydrogen gas. The balanced chemical equation for the reaction is given below. 2 Al(s) + 6 HCl(aq) 2 AlCl 3 (aq) + 3 H 2 (g) If 35.5 mL of...
-
Need help with the incorrectanswer. I have tried 6,750, 81,000, and 0, but all areincorrect. Exercise 4-23 (Algorithmic) (LO. 4) Casper and Cecile divorced in 2018. As part of the divorce settlement,...
-
Fred Barone is describing budgetary control. What steps should be included in Freds description?
-
Let S2X and S2Y be the respective variances of two independent random samples of sizes n and m from N(μX, Ï2X) and N(μY, Ï2Y). Use the fact that F =...
-
22. Repeat the previous problem calculating prices for American options instead of European. What happens?
-
Using the following accounts, prepare a classified balance sheet at year end, May 31, 2014: Accounts Payable, $1,600; Accounts Receivable, $2,200; Accumulated DepreciationEquipment, $1,400; Cash,...
-
I WILL Question 14 2 pts The income statement reveals resources and equities of a firm at a point in time, O resources and equities of a firm for a period of time onet earnings (net income) of a firm...
-
You work in a semiconductor production plant that relies on several chlorofluorocarbons in its manufacturing process. One day, you find an unlabeled gas cylinder, and you are assigned to figure out...
-
A number of compounds containing the heavier noble gases, and especially xenon, have been prepared. One of these is xenon hexafluoride (XeF 6 ), which can be prepared by heating a mixture of xenon...
-
Suppose four gas molecules are placed in the left flask in Figure 19.6(a). Initially, the right flask is evacuated and the stopcock is closed. (a) After the stopcock is opened, how many different...
-
The following information about the payroll for the week ended December 30 was obtained from the records of Saine Co.: Salaries: Sales salaries Deductions: $180,000 Income tax withheld $65,296...
-
You have just been hired as the chief executive officer (CEO) in a medium-sized organization. The organization is not suffering financially, but neither is it doing as well as it could do. This is...
-
The following is the selling price and cost information about three joint products: X Y Z Anticipated production 1 2 , 0 0 0 lbs . 8 , 0 0 0 lbs . 7 , 0 0 0 lbs . Selling price / lb . at split - off...
-
calculate the maximum bending compressive stress of the following section under NEGATIVE bending moment of 216KN.m. 216mm 416mm 316mm 115mm
-
Need assistance with the following forms: 1040 Schedule 1 Schedule 2 Schedule C Schedule SE Form 4562 Form 8995 Appendix B, CP B-3 Christian Everland (SS number 412-34-5670) is single and resides at...
-
At December 31, 20X4, Lake Air Mall, Inc., reported stockholders' equity as follows: Common stock, $1 par, 500,000 shares authorized, 320,000 shares issued ........................ $ 320,000...
-
In the circuit shown in Figure 4, a battery supplies a constant voltage of 40 V, the inductance is 2 H, the resistance is 10, and l(0) = 0. (a) Find l(t). (b) Find the current after 0.1s.
-
Compare the electrostatic potential maps for cycloheptatrienone and cyclopentadienone. Both of these maps were created using the same color scale so they can be compared. Notice the difference...
-
What is the advantage of a differential scanning calorimeter over a bomb calorimeter in determining the enthalpy of fusion of a series of samples?
-
You wish to measure the heat of solution of NaCl in water. Would the calorimetric technique of choice be at constant pressure or constant volume? Why?
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


