As part of its analytical capabilities, the Curiosity Mars rover carries a novel instrument called ChemCam. Do
Question:
As part of its analytical capabilities, the Curiosity Mars rover carries a novel instrument called ChemCam. Do a web search to learn what ChemCam does and how it works and write a short paragraph comparing ChemCam to an AAS instrument like the one shown in Figure 6.1.
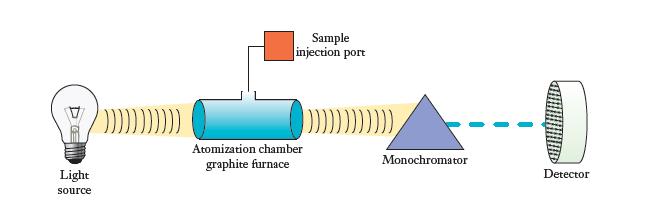
Transcribed Image Text:
DE Light source Sample injection port Atomization chamber graphite furnace Monochromator D Detector
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
ChemCam is a laserinduced breakdown spectroscopy LIBS instrument that is part of the Curiosity Mars rovers analytical suiteIt uses a highpowered laser to vaporize a small spot on a rock or soil sample...View the full answer

Answered By

Irfan Ali
I have a first class Accounting and Finance degree from a top university in the World. With 5+ years experience which spans mainly from the not for profit sector, I also have vast experience in preparing a full set of accounts for start-ups and small and medium-sized businesses. My name is Irfan Ali and I am seeking a wide range of opportunities ranging from bookkeeping, tax planning, business analysis, Content Writing, Statistic, Research Writing, financial accounting, and reporting.
4.70+
249+ Reviews
530+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Which is a useful tool for visualization while reflecting on one's own conceptual framework about technology? a. A Venn diagram b. A summative evaluation in writing c. A survey of interests and...
-
Naturally occurring silicon consists of three isotopes with the following isotopic masses and abundances. Abundance (%) 92.2297 4.6832 3.0872 Isotope 28 Si Isotopic mass (u) 27.9769265327 28.97649472...
-
Find keys K such that DESK (DESK (x)) = x, for all x Such a key is sometimes called a weak key. How many weak keys can you find? To solve this problem you need to look up the exact key schedule...
-
As sales manager, Joe Batista was given the following static budget report for selling expenses in the Clothing Department of Soria Company for the month of October. As a result of this budget...
-
The current risk-free rate of return, rRF, is 4 percent and the market risk pre-mium, RPM, is 5 percent. If the beta coefficient associated with a firm's stock is 2.0, what should be the stock's...
-
8. Suppose call and put prices are given by Strike 50 55 Call premium 9 10 Put premium 7 6 What no-arbitrage property is violated? What spread positionwould you use to effect arbitrage? Demonstrate...
-
Refer to the data for Provost Industries in Case 419. Assume that the company uses the FIFO method in its process costing system. Required: 1. Prepare a report for the Finishing Department for April...
-
The income statement and additional data of Vitamins Plus, Inc. follows: (Click the icon to view the income statement.) Click the icon to view the additional data.) Prepare Vitamins Plus's statement...
-
Write all of the allowed sets of quantum numbers (n, and m ) for a 3p orbital. Strategy The 3p designation tells us the values of the n and, quantum numbers, and we can use the relationship...
-
The fluorescence emitted in XRF is also in the X-ray range of the spectrum, but is always at lower energies than the X-rays used to initiate the process. Explain why this must be true.
-
Abbott Laboratories reports its 50% joint venture investment in TAP Pharmaceutical Products Inc. using the equity method of accounting in its 2007 10-K. The Abbott balance sheet reports an investment...
-
Spitfire Company makes and sells three products: A, B, and C. The following data relate to these products: A B Demand in units Selling price per unit 110 100 90 $180 $210 $195 Raw material costs per...
-
NCF & Partners (NCF) is a firm of CPAslocated in Whitby that has been in business for 20 years. NCF's revenue has declined steadily over the past few years. The partners are looking for ways...
-
Task 4.2Written report Describe how you will present the menu to customers, for example, folders, covers, boards or binding. Include details of colour schemes, pictures, icons, logos, symbols and...
-
The American company "Amazonian", leader in food distribution, is starting operations in Brazil. They just hired a group of new managers who will lead several branches of the company in different...
-
1; Assume you are in charge of fundraising for an organization on your campusa social fraternity or sorority, a business fraternity, or any other such organization. It is your job to identify a...
-
The accounting records of North Central Distributors, Inc., reveal the following: Required Compute cash flows from operating activities by the indirect method. Use the format of the operating...
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Given its mission of providing information to the world, should Google censor searches in China?
-
Identify how IR spectroscopy might be used to monitor the progress of each of the following reactions. a. b. c. d. e. H2 Pt [0]
-
Identify the characteristic signals that you would expect in the diagnostic region of an IR spectrum of each of the following compounds. a. b. c. d. e. f. OH
-
Identify the molecular formula for each of the following compounds, and then predict the mass of the expected molecular ion in the mass spectrum of each compound. a. b. c. d. e.
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


