Calcium carbonate (limestone, CaCO 3 ) dissolves in hydrochloric acid, producing water and carbon dioxide. An unbalanced
Question:
Calcium carbonate (limestone, CaCO3) dissolves in hydrochloric acid, producing water and carbon dioxide. An unbalanced net ionic equation for this reaction is given below. Balance it.
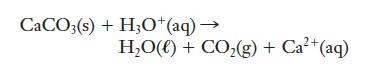
Transcribed Image Text:
CaCO3(s) + H3O+ (aq) → H,O() + CO,(g)+Ca+(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Balanced chemical equation ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Calcium carbonate (limestone, CaCO 3 ) dissolves in hydrochloric acid, producing water and carbon dioxide as shown in the following unbalanced net ionic equation: Suppose 5.0 g of CaCO 3 is added to...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
9. If a two-dimensional array is declared as int M[3][2]; and it has been initialized to all zero's, what will be the contents of this two-dimensional array after executing the code fragment below...
-
A 10.231-g sample of window cleaner containing ammonia was diluted with 39.466 g of water. Then 4.373 g of solution were titrated with 14.22 mL of 0.1063 M HCl to reach a bromocresol green end point....
-
A firm wishing to evaluate interest rate behavior has gathered data on the nominal rate of interest and on inflationary expectations for five U.S. Treasury securities, each having a different...
-
Write a program that lets the user enter the loan amount and loan period in number of years and displays the monthly and total payments for each interest rate starting from 5% to 8%, with an...
-
What role do product costs play in bids? lop5
-
1. What conclusion(s) are indicated by the ratio analysis? 2. What is the firms current payout ratio compared to its historical payout ratio? 3. What are the annual growth rates in the earnings per...
-
You can continue to record depreciation on a fixed asset as long as the asset is still being used in the business. Answers: This statement is true. This statement is false. This statement is...
-
Methyl cyanoacrylate is the chemical name for the substance sold as Super Glue, and it has the chemical formula C 5 H 5 NO 2 . Calculate the number of molecules of this substance in a 1.0-ounce tube...
-
Many chemical reactions take place in the catalytic converter of a car. In one of these reactions, nitric oxide (NO)reacts with ammonia (NH 3 ) to give nitrogen (N 2 ) and water. Write a balanced...
-
Evaluate the iterated integral. /2 (2x (x+z cos(x 2y + z) dy dz dx
-
For each of the matrices determine the value(s) of c for which the given matrix is not invertible. [4 25. 26. 3 5 } ] 6 27. 28. 2 c+4 C -8 c-6]
-
Spitfire Company makes and sells three products: A, B, and C. The following data relate to these products: A B Demand in units Selling price per unit 110 100 90 $180 $210 $195 Raw material costs per...
-
NCF & Partners (NCF) is a firm of CPAslocated in Whitby that has been in business for 20 years. NCF's revenue has declined steadily over the past few years. The partners are looking for ways...
-
Task 4.2Written report Describe how you will present the menu to customers, for example, folders, covers, boards or binding. Include details of colour schemes, pictures, icons, logos, symbols and...
-
The American company "Amazonian", leader in food distribution, is starting operations in Brazil. They just hired a group of new managers who will lead several branches of the company in different...
-
You have the following data for the last 12 months' sales for the PRQ Corporation (in thousands of dollars): a. Calculate a 3-month centered moving average. b. Use this moving average to forecast...
-
A city maintains a solid waste landfill that was 12 percent filled at the end of Year 1 and 26 percent filled at the end of Year 2. During those periods, the government estimated that total closure...
-
Draw a mechanism for each of the following transformations: a. b. c. ,t Dilute H,SO,
-
In this problem, you calculate the error in assuming that ÎH o R is independent of T for the reaction 2CuO(s) 2Cu(s) + O 2 (g). The following data are given at 25°C:
-
If an alkene is protonated and the solvent is an alcohol rather than water, a reaction takes place that is very similar to acid-catalyzed hydration, but in the second step of the mechanism the...
-
selected transactions for cullumber corporation during its first month in business are presented below
-
Questions: President Trump has raised tariffs on steel and aluminum imported into the United States. Answer both of the following: 1) What exporting countries are affected and how large are the...
-
Present Value of Bonds Payable; Premium Moss Co. issued $130,000 of five-year, 11% bonds, with interest payable semiannually, at a market (effective) interest rate of 10%. Determine the present value...

Study smarter with the SolutionInn App


