Certain elements in the periodic table shown in Figure 7.7 had no electronegativity value defined. Based on
Question:
Certain elements in the periodic table shown in Figure 7.7 had no electronegativity value defined. Based on the definition of electronegativity and the identity of these elements, hypothesize as to why they have no electronegativity value.
Figure 7.7
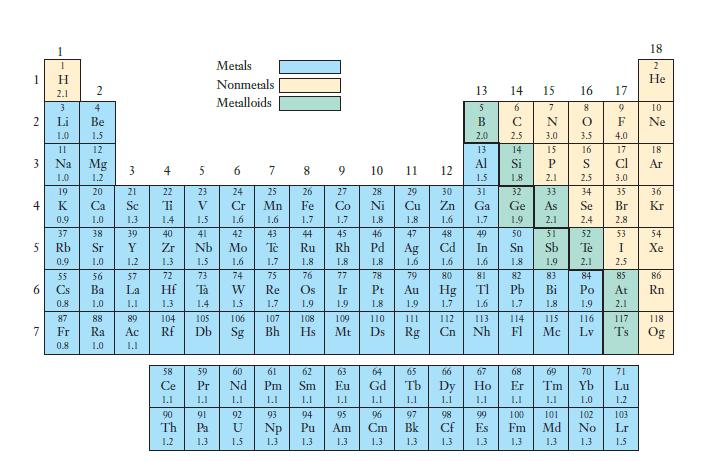
Transcribed Image Text:
1 2 3 4 5 6 7 T H 2.1 3 Li 1.0 11 0.9 55 2 4 Be 1.5 12 Mg 1.2 20 1.0 Ra 1.0 ارا 22 Ti 1.3 1.4 39 Y 1.2 57 La J.L 89 4 23 5 23 V 1.5 40 41 Zr Nh 1.3 1.5 72 73 Ta 1.4 105 Db 1.1 90 91 Th Pa 1.2 1.3 Metals Nonmetals Metalloids. 6 7 24 25 Cr Mn 1.6 1.6 42 43 Mo To 1.6 1.7 74 75 W 15 60 Nd 1.1 92 U 1.3 9 27 Co 542 1.8 76 77 Ir 1.9 10 429 78 Pt 1.8 109 110 Mt Ds 79 1.1 98 CH 1.3 13 5 B 2.0 14 15 6 C 2.5 13 14 Al Si 1.5 1.8 31 32 Ga Ge 1.7 1.9 49 In 1.6 81 F992 67 Ho 1.1 99 Es 13 N 3.0 15 P 2.1 33 1.8 1.9 82 83 Pb Bi 1.7 1.8. 68 Er 1.1 100 Fm 1.3 114 115 Fl Mc 16 8 O 2.4 52 Te 4.0 17 Cl 3.0 35 Br 2.8 53 2.1 2.5 84 85 71 Lu 1.2 103 Lr 1.5 18 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Electronegativity is a measure of an atoms ability to attract electrons in a chemical bondIt is a re...View the full answer

Answered By

Aqib Parvej
I am teaching since my graduation time so I have teaching experience of about 5 years and in these years I learn to teach in the best and interesting way .
4.80+
20+ Reviews
41+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The two employees had an altercation on the job site, one used a blunt instrument to inflict a wound on the other employee. the company terminated one employee and suspended the other. The Union...
-
Elements above uranium in the periodic table do not exist in any appreciable amounts in nature because they have short half-lives. Yet there are several elements below uranium in atomic number with...
-
Use the information in Figure to answer the following questions: a. What is the six-month forward rate for the Japanese yen in yen per U.S. dollar? Is the yen selling at a premium or a discount?...
-
What are the six major factors that distinguish multinational financial management from financial management as practiced by a purely domestic firm? Citrus Products Inc. is a medium-sized producer of...
-
Will your flight get you to your destination on time? The U.S. Bureau of Transportation Statistics reported the percentage of flights that were delayed each month from 1994 through October of 2013....
-
What are the differences in accounting for a forward contract used as a cash flow hedge of (a) a foreign currency denominated asset or liability and (b) a forecasted foreign currency transaction? LO9
-
Solar Energy Corp. has $4 million in earnings with four million shares outstanding. Investment bankers think the stock can justify a P/E ratio of 21. Assume the underwriting spread is 5 percent. What...
-
F5k8-2n Company uses a job-order costing system and applies overhead costs to jobs using a pre-determined overhead rate of 190% of direct materials cost . F5k8-2n Company began work on four jobs...
-
How is electronegativity defined?
-
How does the bond energy of a double bond compare to that of two single bonds between the same elements? How does this relationship explain the types of reactions that compounds with double bonds...
-
Explain how earnings differences could reflect either discrimination or productivity differences.
-
a) Describe the following concepts in the context of organizational development. b) Discuss how these concepts interrelate and support each other within an organizational framework
-
Q2. a) Analyze the importance of communication in the change management process. b) Suggests strategies that a Disaster Management Organization can employ to ensure effective communication during...
-
Q3. a) Explain the following Change Management Models
-
Q3. b) Discuss how each model can be applied in real-world organizational change scenarios.
-
In this question, you will work step-by-step through an optimization problem. A craftsman wants to make a cylindrical jewelry box that has volume, V, equal to 55 cubic inches. He will make the base...
-
Give examples for which machine vision cannot be properly and reliably applied. Explain why machine vision may not be appropriate for these applications?
-
(a) Find the equation of the tangent line to f(x) = x 3 at the point where x = 2. (b) Graph the tangent line and the function on the same axes. If the tangent line is used to estimate values of the...
-
One of the low-energy geometries of digermane, Ge 2 H 2 , is ethene-like. The Lewis dot structure shown
-
S p hybridization on each Ge atom in planar trans-digermane has been described as sp 1.5 for the GeGe sigma bond and sp 1.8 for the GeH bond. Suppose that the Ge lone electron (in terms of Lewis dot...
-
S p hybridization on each Ge atom in planar trans-digermane has been described as sp 1.5 for the GEGe sigma bond and sp 1.8 for the GeH bond. Calculate the HGeGe bond angle based on this...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...
Accounting For Managers And Investors Part 1 Chapter 1-10 2nd Edition - ISBN: 0130079235 - Free Book

Study smarter with the SolutionInn App


