Consider the following thermodynamic data for oxides of manganese. (a) What is the correct chemical nomenclature for
Question:
Consider the following thermodynamic data for oxides of manganese.
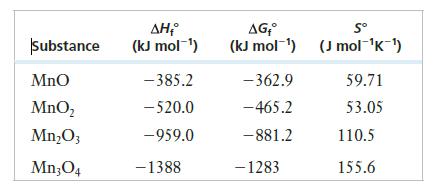
(a) What is the correct chemical nomenclature for each of the first three oxides?
(b) Write and balance chemical equations for the conversion of each of these oxides into Mn3O4.
(c) Based on the free energy changes of these reactions, which oxide is the most stable at room temperature?
Transcribed Image Text:
Substance Mno MnO₂ Mn₂O3 Mn304 ΔΗ (kJ mol-¹) -385.2 - 520.0 -959.0 - 1388 AG₁° Sº (kJ mol-¹) (J mol-¹K-¹) -362.9 - 465.2 -881.2 - 1283 59.71 53.05 110.5 155.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a MnO manganeseII oxide MnO 2 manganeseIV oxide Mn 2 O 3 mangane...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
Reid Corporation's balance sheet at January 1, 20X9 reflected the following balances: Cash & Receivables $ 30,000 Inventory $ 75,000 Land $125,00 Building & Equipment (net) $850,000 Common Stock...
-
The Webster Store shows the following information relating to one of its products. Inventory, January 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . 300 units @ $17.50 Sales, January 8 . ....
-
Make a straight-line depreciation schedule for an asset that costs $7,500 and has a scrap value of $1,200. The useful life of the asset is eight years.
-
Describe the Plan Project phase of the project life cycle. AppendixLO1
-
You have been assigned the task of evaluating two mutually exclusive projects with the following projected cash flows: If the appropriate discount rate on these projects is 10 percent, which would be...
-
The Company uses a periodic inventory sysytem. Determine the cost assigned to the ending inventory using the specific identification method. Ending inventory consists of 400 units from the April 16...
-
(a) When a chemical bond forms, what happens to the entropy of the system? (b) Thermodynamically, what allows any bond formation to occur? (c) What do your answers to parts (a) and (b) suggest must...
-
Ammonia can react with oxygen gas to form nitrogen dioxide and water. (a) Write a balanced chemical equation for this reaction. (b) Use tabulated data to determine the free energy change for the...
-
What other internal resources might NP Hudson access for support, recommendations, and team piloting of her panel?
-
Given below is some is a comparison of financial performance data of a project when flexibility is incorporated (I.e. flexible project) in comparison to when it is not. (i.e. inflexible project) The...
-
For Service Zone H, assuming your shipment chargeable weight is between 100 and 300 kg, at what weight does it become cheaper to declare the shipment weight to be 300 kg.? EG: What is the rate break...
-
Gold Dust Ltd has produced the following budgeted data for its current financial year:- Sales 2900000 Direct materials 400000 Direct labour 500000 Production overhead 1200000 Production cost 2100000...
-
Critical Review V Hide Assignment Information Instructions Williams, A. (2012). Worry, intolerance of uncertainty, and statistics anxiety. Click on the following link to retrieve the article....
-
(4.) Octopussy Company uses a predetermined overhead rate in applying overhead to production orders on a labor-cost basis for Dept. A and on a machine-hour basis for Dept. B. At the beginning of...
-
For the CDMA system in Problem 11.3.8, we wish to use MATLAB to evaluate the bit error rate (BER) performance of the decorrelater introduced Problem 11.3.9. In particular, we want to estimate Pe, the...
-
If there is an unrealized holding gain on available-for-sale investments, it is reported as?
-
Consider the first-order correction to the energy of interacting spins illustrated in Example Problem 28.3 for 2 . Calculate the energy correction to the wave functions 1 = (1) (2), 2 = (1) (2),...
-
What is the difference between a configuration and a permutation?
-
What are the elements of a probability model, and how do they differ for continuous and discrete variables?
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


