Consider the Lewis structure below. Is this an ion? If so, what is its charge? == :O:
Question:
Consider the Lewis structure below. Is this an ion? If so, what is its charge?
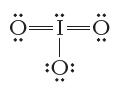
Transcribed Image Text:
ة==ة :O:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
No the Lewis structure you sent is not an ion It is a molecule of ozone O3 An ion is an atom or mole...View the full answer

Answered By

Amit Kumar
I am a student at IIT Kanpur , which is one of the prestigious colleges in INDIA.
Cleared JEE Advance in 2017.I am a flexible teacher because I understand that all students learn in different ways and at different paces. When teaching, I make sure that every student has a grasp of the subject before moving on.
I will help student to get the basic understanding clear. I believe friendly behavior with student can help both the student and the teacher.
I love science and my students do the same.
4.90+
44+ Reviews
166+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Consider the Lewis structure for glycine, the simplest amino acid: (a) What are the approximate bond angles about each of the two carbon atoms, and what are the hybridizations of the orbitals on each...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Briefly describe some common information system controls that need to be implemented by business managers, not IS professionals.
-
rn rn Anushka (an Australian Tax resident) works as an employee for a child care centre, Brilliant Kids Pty Ltd, on a permanent part-time basis while she runs her business as a day carer for her own...
-
What is a real option? What are some types of real options? Shrieves Casting Company is considering adding a new line to its product mix, and the capital budgeting analysis is being conducted by...
-
How does direct exporting differ from indirect exporting?
-
What arrangements are required for payments during construction?
-
Cite the advantages and disadvantages associated with these merchandise sources for your regular fast-food outlet. How would your answers differ for a global apparel chain? a. Company-owned. b....
-
Data related to the acquisition of timber rights and intangible assets during the current year ended December 3 1 are as follows: A . Timber rights on a tract of land were purchased for $ 3 , 5 8 9 ,...
-
The following molecules have similar formulas, but each has a different shape. Draw Lewis structures for these compounds and then determine their molecular shapes. (a) SiF 4 , (b) KrF 4 , (c) SeF 4
-
The N 5 + cation has been synthesized and studied. Consider the possible Lewis structure below. Indicate the hybridization expected for each nitrogen atom and the expected bond angles. Assuming that...
-
Refer to Section 6.4.1. When Y is N( i , 2 ), consider the comparison of ( 1 ,...., 1 ) based on independent samples at the I categories of X. When approximately i = + x i , explain why the t or F...
-
5.Descibe the HSI color image model 6. Describe the basic relationship between the pixels
-
1. What is the need for transform? 2. What is Image Transform? 3. What are the applications of transform? 4. Give the Conditions for perfect transform . 5. What are the properties of unitary...
-
6. Define Fourier transform pair 7. Define Fourier spectrum and spectral density 8. Give the relation for 1-D discrete Fourier transform pair 9. Specify the properties of 2D Fourier transform. 10....
-
16. What is wrap around error? 17. Give the formula for correlation of 1D continuous function. 18. What are the properties of Haar transform. 19. What are the Properties of Slant transform 20....
-
21. Define fast Walsh transform. 22. Give the relation for 1-D DCT. 23. Write slant transform matrix SN. 24. Define Haar transform. 25. Define K-L transform. 26. Give the equation for singular value...
-
Would machining centers be suitable for just-in-time production? Explain.
-
How many years will it take a $700 balance to grow into $900 in an account earning 5%?
-
List the allowed quantum numbers m l and m s for the following subshells and determine the maximum occupancy of the subshells: a. 2p b. 3d c. 4 f d. 5g
-
The effective path length that an electron travels before being ejected into the vacuum is related to the depth below the surface at which it is generated and the exit angle by d = cos , where is...
-
The inelastic mean free path of electrons in a solid, , governs the surface sensitivity of techniques such as AES and XPS. The electrons generated below the surface must make their way to the surface...
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


