Determine the shape of each of the following species. (a) P O 4 3 , (b) PCl
Question:
Determine the shape of each of the following species.
(a) P O 4 3–,
(b) PCl5
Strategy Draw the Lewis structures. Each of these molecules has only single bonds around its central atom. So we can count the number of bonding pairs around the central atom and assign the geometry by consulting Table 7.3 as needed.
Table 7.3
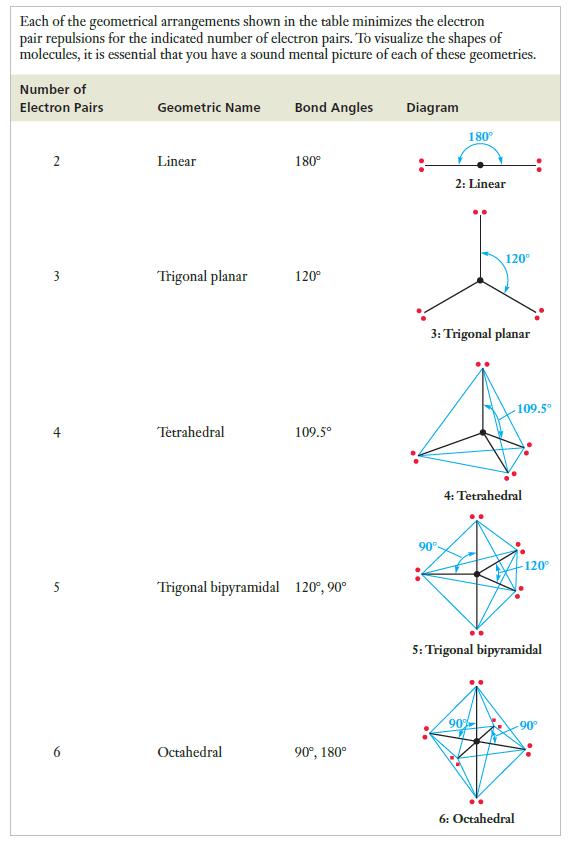
Transcribed Image Text:
Each of the geometrical arrangements shown in the table minimizes the electron pair repulsions for the indicated number of electron pairs. To visualize the shapes of molecules, it is essential that you have a sound mental picture of each of these geometries. Number of Electron Pairs 2 3 5 6 Geometric Name Linear Trigonal planar Tetrahedral Bond Angles Octahedral 180° 120° 109.5⁰ Trigonal bipyramidal 120°, 90° 90⁰, 180° Diagram 180° 90° 2: Linear 3: Trigonal planar 120⁰ 4: Tetrahedral 90% 109.5° 5: Trigonal bipyramidal 6: Octahedral -120° -90°
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a The Lewis structure of P O 4 3 which we drew in Example Problem 75 is The central phosphorus atom has a complete octet of electrons contributed by f...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Use VSEPR theory to determine the shape of the NOF molecule. Strategy Once again, we start by drawing the Lewis structure. Then count the number of regions of electron density around the central...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The histograms show the number of pairs of shoes reported for 300 males and for 300 females. Descriptive statistics are also shown. a. Describe the shape of each histogram. b. Because of the shapes,...
-
(a) Find the first order fraction transformation where z 1 =,z 2 =0, z 3 =1 is thought to be w 1 =1, w 2 =i, w 3 =-1 each. (b) Find the anchor points of w=(z-1)/(z+1)
-
How would the analysis be different if Hagers intended to recapitalize Lyons with 40% debt costing 10% at the end of four years? This amounts to $221.6 million in debt as of the end of 2013. The...
-
Cecilia and Nathan estimate their total cost for a vacation in Australia to be $14,775. a. What percentage is this cost of their combined gross monthly income of $8775? b. If 72% of their gross...
-
What is the net impact on Werners net income for the quarter ended March 31, 2009, as a result of this forward contract hedge of a firm commitment? LO9 a. $-0-. b. $ 1,250 increase in net income. c....
-
Refer to Problem 10-1. What is the projects PI?
-
What Is the Difference Between Single and Dual-Rate Allocations? And write pros and cons of dual rate allocation Please type the answer on the keyboard
-
Why is the Na 2+ ion not found in nature?
-
Use the concept of polarity of water and the basic composition of the body to explain why the polarity of biomaterials is important.
-
In Exercise 11.17, we ran a multiple regression with Gender as a predictor. Now run separate regressions for males and females. The file at...
-
Exhibit 12: Average Credit Quality Ratios [1] Based on this information you can compare O&Rs financial ratios to the average debt rating ratios above to assess what O&Rs credit rating would be if it...
-
Which of the following statements about QuickBooks Bill Pay are correct? Select all that apply. You can configure QuickBooks Bill Pay to pay bills automatically when they're added to QuickBooks...
-
Ash purchases 500 shares of XYZ for $10/share. Ten months later, when the shares are trading at $15/share, they donate them to Caring Trust, a qualified charity. Three months after the donation is...
-
Bob gets a X = 60 on his psychology exam and a X = 56 on his Biology exam. Psych exam scores had a =50 and =10 while Bio exam scores had a =48 and =4. Both professors grade on a curve. 1 - For which...
-
1. Lucky Company's direct labor information for the month of February is as follows: Actual direct labor hours worked (AQ) 61,500 Standard direct labor hours allowed (SQ) 63,000 Total payroll for...
-
It has been commonly acknowledged that, at the early stages of development and implementation of industrial robots, the usefulness and cost effectiveness of the robots were overestimated. What...
-
For the data in Exercise 17-19, use the FIFO method to summarize total costs to account for, and assign these costs to units completed and transferred out, and to units in ending work in process....
-
Hydroxymethylene has never actually been observed, although it is believed to be an intermediate both in the photo-fragmentation of formaldehyde to hydrogen and carbon monoxide, and in the...
-
The three vibrational frequencies in H 2 O (1595, 3657, and 3756 cm 1 ) are all much larger than the corresponding frequencies in D 2 O (1178, 2671, and 2788 cm 1 ). This follows from the fact that...
-
DielsAlder reactions commonly involve electron-rich dienes and electron-deficient dienophiles: The rate of these reactions generally increases with the Ï -donor ability of the diene substituent,...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


