Question: In a process called flash electroplating, a current of 2.50 10 3 A passes through an electrolytic cell for 5.00 minutes. How many moles
In a process called flash electroplating, a current of 2.50 × 103 A passes through an electrolytic cell for 5.00 minutes. How many moles of electrons are driven through the cell?
Strategy
Because we know both the current and the time for which it was applied, we can use the relationship between charge and current in Equation 13.8 to obtain the charge. Then, Faraday’s constant will let us convert that charge into moles of electrons. We must be careful with units, however, because the ampere is charge per second and time is given here in minutes.
Equation 13.8
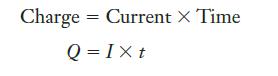
Charge Current X Time Q=Ixt =
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Discussion This twostep manipulation is really a stoichiometry problem as it allows us to find ... View full answer

Get step-by-step solutions from verified subject matter experts


