Look at the table of electron configurations in Appendix c. Which elements have configurations that are exceptions
Question:
Look at the table of electron configurations in Appendix c. Which elements have configurations that are exceptions to the aufbau principle? Propose a reason why these elements have these exceptions.
Data from appendix c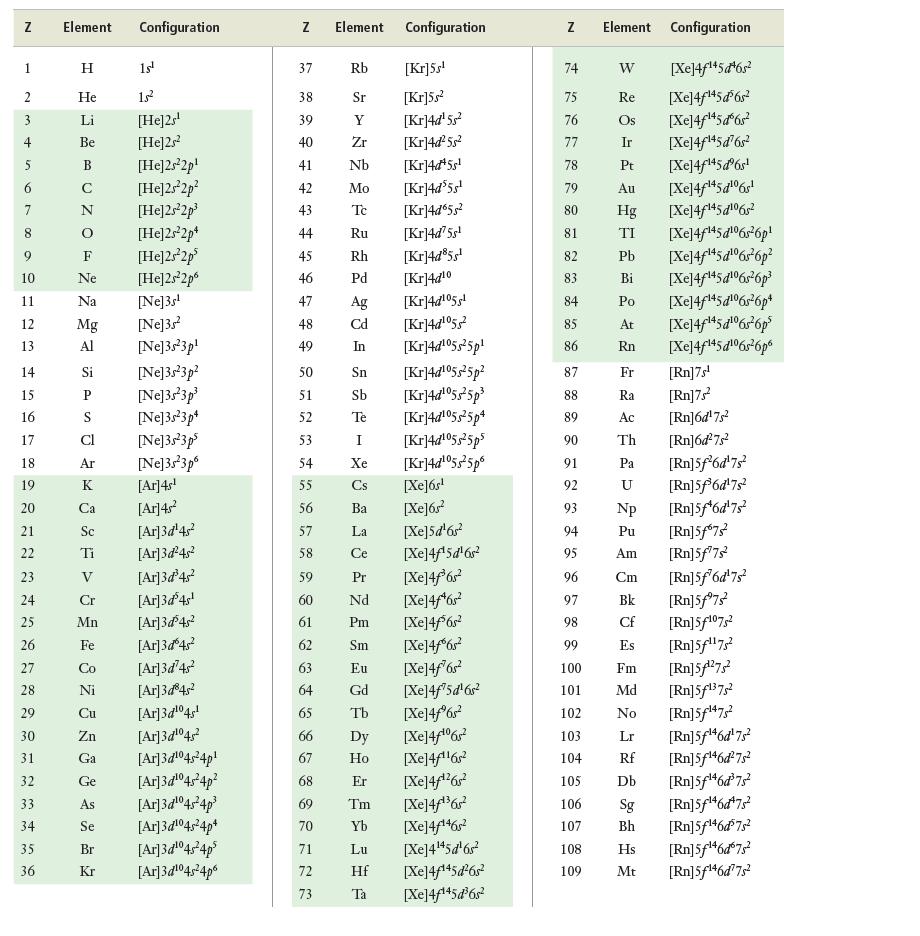
Transcribed Image Text:
N 1 2 + 5 6 7 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Element Configuration H 15¹ He 15² Li [He]2s¹ Be [He]2s² [He]2s²2p¹ [He]2s²2p² [He]2s²2p³ [He]2s²2p* [He]2s²2p³ [He]2s²2p6 [Ne]3s¹ [Ne]3s² [Ne]3s²3p¹ AUZO Z Z Ne Na Mg Al Si P S Ar K Ca Sc Ti V Cr Mn Fe Co [Ne]3s²3p² [Ne]3s 3p³ [Ne]3s²3p4 [Ne]3s 3p5 [Ne]3s²3p6 [Ar]4s¹ [Ar]4s² [Ar]3d¹4s² [Ar]3d²4s² [Ar]3d³4s² [Ar]3d³4s¹ [Ar]3d³45² [Ar]3d 4s² [Ar]3d¹4² Ni [Ar]3d³45² Cu [Ar]3d¹⁰45¹ Zn [Ar]3d¹⁰45² Ga [Ar]3d¹04s²4p¹ Ge [Ar]3d¹⁰45²4p² As [Ar]3d¹04s²4p³ Se [Ar]3d¹04s²4p+ Br [Ar]3d¹04s²4p³ Kr [Ar]3d¹04s²4p6 N 37 38 39 40 41 42 43 44 45 46 7 48 49 50 47 50 Element Configuration 72 75 Rb [Kr]5s¹ Sr [Kr]55² Y [Kr]4d¹5s² Zr [Kr]4d²5s² Nb [Kr]4d¹5s¹ Mo [Kr]4d³5s¹ Tc [Kr]4d65s² Ru [Kr]4d' 5s¹ Rh [Kr]4d³5s¹ Pd [Kr]4d10 [Kr]4d¹05¹ [Kr]4d¹055² [Kr]4d¹055²5p¹ [Kr]4d¹05s²5p² [Kr]4d¹05s²5p³ [Kr]4d¹05525p4 [Kr]4d¹05s²5ps [Kr]4d¹055²5p6 73 Ag Cd In 51 52 53 54 55 56 Ba 57 La 58 Ce 59 Pr 60 Nd 61 Pm 62 Sm 63 Eu 64 65 Sn Sb Te [Xe]6s¹ [Xe]6s² [Xe]5d¹6s² [Xe]4f¹5d¹6s² [Xe]4far [Xe]4f¹6s² [Xe]4f6r2 [Xe]4f6s? [Xe]4f762 [Xe]4f¹5d¹6s² [Xe]4f®6? 66 Dy [Xe]4/06r 67 Ho [Xe]4f1162 68 Er [Xe]4f*26r? 69 Tm [Xe]4f36r2 70 Yb [Xe]4f1462 71 Lu [Xe]4145d6r Hf [Xe]4f¹45d²6s² Ta [Xe]4f¹45d³6s² I Xe Cs Gd Tb Z 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 Element W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Rf Db Configuration [Xe]4f145 đ6g? [Xe]4f14562 [Xe]4/145d6s? [Xe]4f145d6s? [Xe]4f¹45dº6s¹ [Xe]4f**5¢06r! [Xe]4f145g16g? Sg Bh [Xe]4f45d!06r6p! [Xe]4f**5d!06r6p2 [Xe]4f145¢106s6p* [Xe]4f145¢106s26p* [Xe]4f**5¢!6r6p* [Xe]4f**5d106r6p Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md [Rn]5f¹³75² No [Rn]5f¹47² Lr [Rn]5f¹46d¹75² [Rn]5f¹46d²75² [Rn]5f¹46d³75² [Rn]5f¹46d75² [Rn]5f¹46d³75² Hs [Rn]5f¹46d675² Mt [Rn]5f¹46d775² [Rn]7s¹ [Rn]7s² [Rn]6d¹7s² [Rn]6d²7s² [Rn]5f6d¹7s [Rn]5f³6d¹7s² [Rn]5f¹6d¹7s² [Rn]5f673² [Rn]5f¹7s² [Rn]5f6d¹7s² [Rn]5f7s² [Rn]5f¹07,² [Rn]5f¹¹75² [Rn] 5¹27²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The elements that have configurations that are exceptions to the ...View the full answer

Answered By

Ariz Azad
I love explaining things to people. Sharing my knowledge with others is one of the rare things that gives me immense pleasure. I have been a tutor on Chegg for more than a year now for Finance. Nothing beats the satisfaction of getting a thumbs up from students for your answer. Maintaining quality in teaching is one of the things I have learned through experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
The CICA Handbook is available to most post-secondary students through their institutions subscription. Visit edu.knotia.ca to complete this exercise. (You may need to complete this exercise on...
-
Look again at the table of post-graduation plans for the senior class in Exercise 27. a) Find the conditional distributions (percentages) of plans for the white students. b) Find the conditional...
-
Write a C++ program with recursion to print the following pattern for any odd number greater than or equal to 5. Note: the last row must be filled with digits from your ID in case the pattern is...
-
Presto Corp. has collected the following data concerning its maintenance costs for the past 6 months. Compute the variable and fixed cost elements using the high-lowmethod. July August September...
-
McCormick & Company, Inc. is one of the worlds leading producers of spices, herbs, seasonings, condiments, and other flavorings for foods. Its products are sold to consumers, with some of the leading...
-
7. Using the information in Table 1, a. Compute the implied forward rate from time 1 to time 3. b. Compute the implied forward price of a par 2-year coupon bond that will be issued at time 1.
-
Air travel on Mountain Airlines for the past 18 weeks was: a. Explain why an averaging technique would not be appropriate for forecasting. b. Use an appropriate technique to develop a forecast for...
-
Ho News Report Group has two major divisions: print and Internet. John Mendenhall, CEO of News Report Group, soeke your advice on revising the existing bonus plan for division managers of News Report...
-
Distinguish between the terms core electrons and valence electrons.
-
From the list of atoms and ions given, identify any pairs that have the same electron configurations and write that configuration: Na + , S 2-, Ne, Ca 2+ , Fe 2+ , Kr, I - .
-
Account payable are sometimes referred to as .
-
Your friend Amber has approached you seeking advice concerning two investment opportunities that she is presently considering. Her classmate Simone has asked her for a loan of $5,000 to help...
-
Please read the following carefully. For each question on the exam, you should assume that: 1. unless expressly stated to the contrary, all events occurred in ?the current taxable year;? 2. all...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Can I get clear explanation how to work these. Thanking you in advance. 1. A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 uC. Determine the magnitude and direction of the...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
Refer to Under Armour's balance sheet reproduced in the chapter. Required In which of the assets would you expect Under Armour's land to be included? What does this amount represent (i.e., cost,...
-
Separate variables and use partial fractions to solve the initial value problems in Problems 18. Use either the exact solution or a computer-generated slope field to sketch the graphs of several...
-
Identify which of the following compounds is expected to have the larger heat of combustion:
-
Draw each of the following compounds: (a) 2,2,4-Trimethylpentane (b) 1,2,3,4-Tetramethylcycloheptane (c) 2,2,4,4-Tetraethylbicyclo [1.1.0] butane
-
Sketch an energy diagram that shows a conformational analysis of 2,2-dimethylpropane. Does the shape of this energy diagram more closely resemble the shape of the energy diagram for ethane or for...
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


