Many mining operations produce tailings that can be oxidized to form acids, including sulfuric acid. One chemical
Question:
Many mining operations produce tailings that can be oxidized to form acids, including sulfuric acid. One chemical that reacts in this way is chalcopyrite, CuFeS2, whose net oxidation can be described by the following equation:
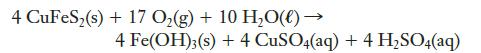
In a laboratory experiment to study weathering, a mining engineer put a 2.3-kg sample of CuFeS2(s) near an empty 40-L container. Simulated weathering experiments were conducted and the runoff was collected, leading to 35.8 L of solution. A 25.00-mL aliquot of this solution was analyzed with 0.0100 M NaOH(aq). The titration required 38.1 mL to reach the equivalence point.
(a) What was the concentration of sulfuric acid?
(b) Assuming the only acid in the container comes from the reacted chalcopyrite, what mass of the chalcopyrite has reacted?
(c) What percentage of the chalcopyrite reacted in this experiment?
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





