Methane can be produced from CO and H 2 . The process might be done in two
Question:
Methane can be produced from CO and H2. The process might be done in two steps, as shown below, with each step carried out in a separate reaction vessel within the production plant.
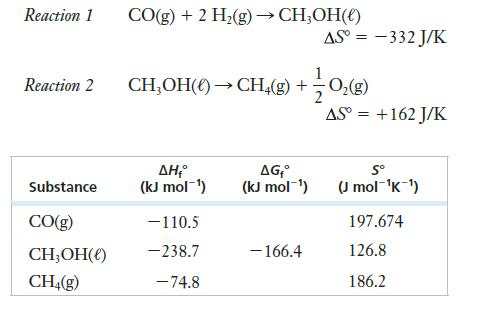
You should be able to work this problem without using any additional tabulated data.
(a) Calculate ΔH° for reaction 1.
(b) Calculate ΔGf° for CO(g).
(c) Calculate S° for O2(g).
(d) At what temperatures is reaction 1 spontaneous?
(e) Suggest a reason why these two steps would need to be carried out separately.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





