The following equilibrium is established in a closed container: Set up a table like the one that
Question:
The following equilibrium is established in a closed container:
![]()
Set up a table like the one that follows and indicate whether equilibrium concentrations increase, decrease, or remain the same.
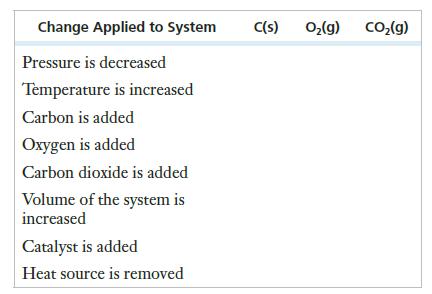
Transcribed Image Text:
C(s) + O₂(g) → CO₂(g) AH° = -393 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Lets set up a table to analyze the changes in equilibrium concentrations when different conditions a...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The following equilibrium is established in a closed container: How does the equilibrium shift in response to each of the following stresses? (a) The quantity of solid carbon is increased. (b) A...
-
A sample of air with a mole ratio of N 2 to O 2 of 79 : 21 is heated to 2500 K. When equilibrium is established in a closed container with air initially at 1.00 atm, the mole percent of NO is found...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
need help entering sale oof land transaction in intuitproconnect On December 31, 2021, Anthony sold the inherited land from his uncle. The consideration was \( \$ 950,000 \) installment note plus the...
-
List and briefly describe the four parts of typical e-mail messages and memos.
-
Graph the updating functions associated with the following discrete-time dynamical systems, and cobweb for five steps starting from the given initial condition. xt+1 = 4 - xt, starting from x0 = 1...
-
3. A fund used to account for all financial resources except those required to be accounted for in another fund.
-
Peterson Company is preparing the annual financial statements dated December 31, 2010. Ending inventory information about the five major items stocked for regular sale follows: Required: Compute the...
-
There is no other information about this questiononly two multiple choice QUESTIONS 3-4 Questions 3 and 4 refer to the financial data for QAABC Ltd, shown below. QAABC Ltd Account Balances (extract)...
-
An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to capture toxic hydrogen sulfide gases present by reaction with aqueous iron(II) nitrate to form solid...
-
A chemical engineer is working to optimize the production of acrylonitrile to be used in the manufacture of carbon fibers. The reaction being used is the combination of propene gas, ammonia, and...
-
Write a program that enables two users to chat. Implement one user as the server (Figure 31.21a) and the other as the client (Figure 31.21b). The server has two text areas: one for entering text and...
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
What does this example tell us about product design?
-
The production budget of Artest Company calls for 80,000 units to be produced. If it takes 30 minutes to make one unit and the direct labor rate is $16 per hour, what is the total budgeted direct...
-
If the force applied to the handle of the load binder is 50 lb, determine the tensions T 1 and T 2 in each end of the chain and then draw the shear and moment diagrams for the arm ABC. 50 lb F12 in.-...
-
Draw the shear and moment diagrams for the shaft. The bearings at A and D exert only vertical reactions on the shaft. -15 in.12 in.- 20 in. -14 in- A D 35 lb 80 lb 110 lb
-
The crane is used to support the engine, which has a weight of 1200 lb. Draw the shear and moment diagrams of the boom ABC when it is in the horizontal position. - 3 ft -5 ft- 4 ft
-
C0 = 10.648148 b) ( 4 Marks ) As of now, Given the above conditions on the option, what is the intrinsic value of the call option? What is the time value of the call option?
-
interest revenue 19,500 retained earning,end 5,000 selling expenses 145,00 prepaid insurance 20,000 loss and disposal of a business (discountied),net 28,000 income from operation 140,000 unearned...
-
cost that do not extend the acid capacity or it's useful life, but merely maintained the assd, or restore it to working order are recorded as losses True or False

Study smarter with the SolutionInn App


