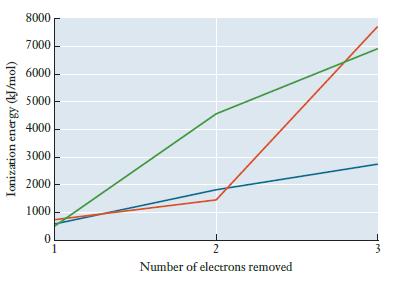
The graph below shows the first three ionization energies for sodium, magnesium, and aluminum. Without consulting a
Question:
The graph below shows the first three ionization energies for sodium, magnesium, and aluminum. Without consulting a list of values, determine which line in the graph corresponds to each element.
Transcribed Image Text:
Ionization energy (kJ/mol) 8000 7000 6000 5000 4000 3000 2000 1000 0 2 Number of electrons removed 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Green ...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
A particular element has the following values for its first four ionization energies: 900, 1760, 14,850, and 21,000 kJ/mol. Without consulting a list of ionization energy values, determine what group...
-
The graph below shows a production possibilities curve for 2014 and two potential production possibilities curves for 2015, denoted 2015A and 2015B. a. Which of the labeled points corresponds to...
-
The graph below shows the hours studied and the test grades on a biology test for 7 students. The red line on the graph can be used to approximate the test grade the average student receives for the...
-
describe Jarir bookstore by providing the followings: - A brief introduction regarding the history of this brand and its main activity? - Describe how and why the brand's activity has evolved through...
-
Stiever Company estimates that variable costs will be 60% of sales, and fixed costs will total $800,000. The selling price of the product is $4. Instructions (a) Prepare a CVP graph, assuming maximum...
-
1. Do you think Thompkinss response of yes to the police inquiry should have been admissible in court against him? Explain. 2. In general, do you think the Miranda warnings offer too much protection...
-
Assume that Hill issues 10,000 shares of common stock with a $5 par value and a $40 fair value to obtain all of Lorings outstanding stock. How much goodwill should be recognized? a. 0. b. $15,000. c....
-
Your investment department has researched possible investments in corporate debt securities. Among the available investments are the following $100 million bond issues, each dated January 1, 2011....
-
Sammy Chum, the owner of SC Limited, prepared the following 2 0 1 9 direct labor budget: \ table [ [ \ table [ [ SC Limited ] , [ Direct Labor Budget ] , [ For the Year Ended November 3 0 , 2 0 1 9 ]...
-
Which graph correctly depicts the first ionization energy of three elements in groups 14 (dashed line) and 17 (solid line)? Explain the reasoning you used to make your choice. 1st I.E. Si Br Ge 1st...
-
Why is there no element to the immediate right of magnesium in the periodic table?
-
What are the main reasons for using cellular systems? How is SDM typically realized and combined with FDM? How does DCA influence the frequencies available in other cells?
-
(25 Points) University Painting is considering investing in a new paint sprayer to allow them to paint more classrooms in less time. The sprayer would have the following cash flow and cost of capital...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Summit Regional Medical Center operates as a private not-for-profit hospital, providing services to a community of 20,000 and the surrounding rural areas. Summit has maintained a banking relationship...
-
Woodland Wearables produces two models of a smart watch, the Basic and the Flash. The watches have the following characteristics: Basic Flash Selling price per watch $ 3 3 0 $ 4 9 0 Variable cost per...
-
Introduction This practice case has been designed to give introductory-level business students practical experience in the application of accounting concepts. This practice case will provide students...
-
After you have gained five years of experience with a large CPA firm, one of your clients, Duke Inc., asks you to take over as chief financial officer for the business. Duke advises its clients on...
-
Use translations to graph f. f(x) = x-/2 +1
-
The molar volume of H 2 O(l) decreases with increasing temperature near 4C. Can you explain this behavior using a molecular level model?
-
For each compound below, identify any polar covalent bonds, and indicate the direction of the dipole moment using the symbols + and -: a. HBr b. HCl c. H 2 O d. CH 4 O
-
Why was the following qualification made in Section 3.7? Note that Equation (3.47) is only applicable to a process in which there is no change in the phase of the system, such as vaporization or...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


