The Solvay process is important in the commercial production of sodium carbonate (Na 2 CO 3 ),
Question:
The Solvay process is important in the commercial production of sodium carbonate (Na2CO3), which is used in the manufacture of most glass. The last step in the Solvay process is the conversion of NaHCO3 (sodium bicarbonate, or baking soda) to Na2CO3 by heating:
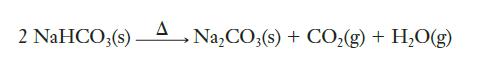
In a laboratory experiment, a student heats 42.0 g of NaHCO3 and determines that 22.3 g of Na2CO3 is formed. What is the percentage yield of this reaction?
Strategy We know the actual yield from the experiment. To calculate the percentage yield, first we need to find the theoretical yield. We can do that by calculating the maximum quantity of product that could form, based on the stoichiometry of the reaction. Once we have both the theoretical yield and the actual yield, finding the percentage yield is straightforward.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





