The vessel on the left contains a mixture of oxygen and nitrogen at atmospheric pressure. The vessel
Question:
The vessel on the left contains a mixture of oxygen and nitrogen at atmospheric pressure. The vessel on the right is evacuated.
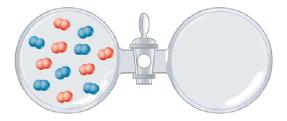
(a) Describe what will happen when the stopcock is opened.
(b) If you could see the individual molecules, what would you observe after a period of time has passed?
(c) Explain your answers to parts (a) and (b) in terms of probabilities.
(d) What is the probability that at any one moment all the oxygen molecules will be in one vessel and all the nitrogen molecules will be in the other? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





