Question: Two weak acids, A and B, are each titrated with the same solution of a strong base, producing the following titration curves. (a) Which acid
Two weak acids, A and B, are each titrated with the same solution of a strong base, producing the following titration curves.
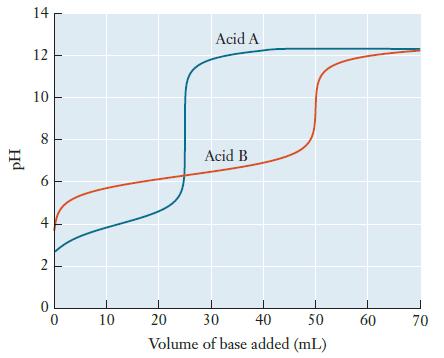
(a) Which acid has a larger Ka value, A or B? Explain.
(b) Use the titration curves to estimate the pH at the equivalence point for each acid. Explain why these values are not the same.
(c) If equal volumes of Acid A and Acid B were used, which one had the higher initial concentration? Explain.
pH 14 12 10 8 6 4 2 0 10 1 20 Acid A Acid B I 50 30 40 Volume of base added (mL) 60 70
Step by Step Solution
3.48 Rating (165 Votes )
There are 3 Steps involved in it
a Halfway to the equivalence point the pH will be equa... View full answer

Get step-by-step solutions from verified subject matter experts


