Use the signs of H and S to explain why ice spontaneously melts at room temperature but
Question:
Use the signs of ΔH and ΔS to explain why ice spontaneously melts at room temperature but not outside on a freezing winter day.
Strategy This problem calls for the same type of reasoning used to construct Table 10.2. We must determine whether the process is endothermic or exothermic and whether it increases or decreases the entropy of the system. Then, by considering the signs of ΔH and ΔS in conjunction with Equation 10.4, we can attempt to explain the behavior described.
Table 10.2
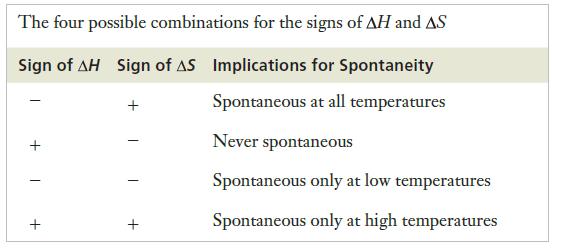
Equation 10.4
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





