Using the equations determine the equilibrium constant for the following reaction: HASO4 (aq) AsO4 (aq) + H+
Question:
Using the equations
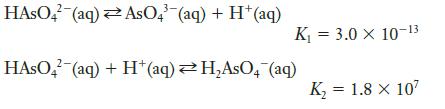
determine the equilibrium constant for the following reaction:
![]()
Transcribed Image Text:
HASO4 (aq) AsO4 (aq) + H+ (aq) K₁ = 3.0 X 10-13 HASO4 (aq) + H+ (aq)H₂AsO4 (aq) K₂ = 1.8 x 10²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To determine the equilibrium constant for the reaction HAsO4aq AsO4aq 2H...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant for the reaction SO3 SO2 + O has the following values: Determine the average heat of dissociation using graphical method. T 800 K 900 K 1000 K 1105K | 0.0319 | 0.153 | 0.540...
-
The following reaction has an equilibrium constant Kc equal to 3.59 at 900oC. For each of the following compositions, decide whether the reaction mixture is at equilibrium. If it is not, decide which...
-
The equilibrium constant for the following reaction is 1.0 Ã 10-3: Cr'. (aq) + H2EDTA2-(aq)--CrEDT A-(aq) + 2H' (aq) CH2-CO2 02GH CH EDTA N-CH,--CH2- O2C-CH2 CHy-CO2-...
-
The combined sewer system in city ABC is comprised of two parallel interceptors referred to as "North" and "South" lines. The southern line is connected to a newly built wastewater treatment plant....
-
Describe the types of cost-based pricing and the methods of implementing each.
-
Find c such that f (x) = -0.2x2 - 3x + c has a maximum value of -225.
-
Hold goods. Goods are kept in storage and under proper protection until needed. LO.1
-
Lovely Lawns, Inc., intends to use sales of lawn fertilizer to predict lawn mower sales. The store manager estimates a probable six-week lag between fertilizer sales and mower sales. The pertinent...
-
n December 31, 2019, the ledger of Lopez Company contained the following account balances: Cash $ 66,000 Maria Lopez, Drawing $ 52,000 Accounts Receivable 5,800 Fees Income 107,500 Supplies 4,200...
-
For each of the following equations, write the equilibrium expression for the reverse reaction. (a) 2 C(s) + O 2 (g) 2 CO(g) (b) AgCl(s) Ag + (aq) + Cl (aq)
-
For the equilibria given below, determine each of the following: (a) Equilibrium expressions for K 1 and K 2 (b) The equation for the reaction that is the sum of the two equations (c) The...
-
Modify Prob. 4.88 so that the outer cylinder also moves to the left at constant speed V. Find the velocity distribution z(r). For what ratio V/U will the wall shear stress be the same at both...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a converging lens, closer to the lens than to the focal point. (b) Is the image...
-
Power efficiency has become very important for modern processors, particularly for embedded systems. Create a version of gcc for two architectures that you have access to, such as x86, RISC-V,...
-
There is a movement toward wireless mobile computing using thin-client technology. Go to the Web and visit some of the ma jor computer vendors that are producing thin-client products such as handheld...
-
Draw a B-tree of order 4 and height 3 containing the fewest elements. Show an example of a split that would be applied by inserting the fewest number of elements.
-
Repeat Example 10-4, except calculate the diameter at the bottom of the column. Example 10-4 A distillation column is separating n-hexane from n-heptane using 1-in. ceramic Intalox saddles. The...
-
Why do large companies like these go to so much trouble to invest in CSR?
-
Define the term utility software and give two examples.
-
The shaft is supported by a smooth thrust bearing at A and a smooth journal bearing at B. Determine the resultant internal loadings acting on the cross section at C. 600 N/m -1 m--1 m---1 m--1.5...
-
Determine the resultant internal loadings acting on section bb through the centroid C on the beam. B' 900 Ib/ft 60 3 ft 30 A 6 ft
-
Determine the resultant internal normal and shear force in the member at (a) section aa and (b) section bb, each of which passes through the centroid A. The 500-lb load is applied along the...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


