100.0 J of heat is added to a 3.45-g sample of an unknown metal. The temperature of...
Question:
100.0 J of heat is added to a 3.45-g sample of an unknown metal. The temperature of the metal increases from 22.37 °C to 54.58 °C. Use the date in Table 5.1 to identify the metal.
Table 5.1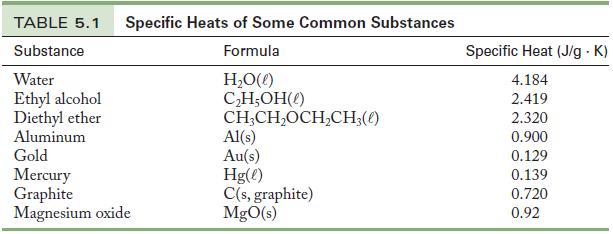
Transcribed Image Text:
TABLE 5.1 Specific Heats of Some Common Substances Substance Formula H₂O(l) C₂H₂OH() CH₂CH₂OCH₂CH3(0) Water Ethyl alcohol Diethyl ether Aluminum Gold Mercury Graphite Magnesium oxide Al(s) Au(s) Hg(e) C(s, graphite) MgO(s) Specific Heat (J/g.K) 4.184 2.419 2.320 0.900 0.129 0.139 0.720 0.92
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
AH100 J mass g345g AT TT5458 C2237 ...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
1. Examine the hanging drop slide and complete the following table with respect to the size, shape, and motility of the different bacteria. Bacterium B subtilis P aeruginosa S volutans Size 2. Draw a...
-
A shareholder made a loan of 1,000 to a corporation in which she owned 50% of the stock. The loan is evidenced by a promissory note with the word "LOAN" printed at the top. The loan has a market...
-
You work for a gas turbine design company and have a client who has a fairly loose specification for a gas turbine engine. You are required to design an aviation gas turbine to power the aircraft...
-
Repeat parts (g) and (i) of Example 17.9 if the solution velocity past the crystal face is reduced from 5 cm/s to 1 cm/s.
-
Peloton Company constructed a building at a cost of $2,400,000 and occupied it beginning in January 2000. It was estimated at that time that its life would be 40 years, with no residual value. In...
-
What conditions must be met to qualify a forward exchange contract as the hedge of a firm foreign-currency-denominated commitment!
-
Hagler Corporation purchased a building by signing a $150,000 long-term mortgage with monthly payments of $1,200. The mortgage carries an interest rate of 8 percent per year. Prepare a monthly...
-
Antioch Company makes eBook readers. The company had the following amounts at the beginning of Year 2 : Cash, $ 6 7 2 , 0 0 0 ; Raw Materlals Inventory, $ 7 1 , 0 0 0 ; Work In Process Inventory, $ 2...
-
Calculate the HPPDs for each of the following Telemetry unit Hours Classifications for one pay period of 14 days. The Budget Average Daily UOS is 20 and the pay period total is 280. Required data is...
-
It takes 677 J of heat to increase the temperature of 25.0 g liquid ethanol (C 2 H 5 OH) from 23.5 C to 34.7 C. What is the specific heat of this substance?
-
What mass of acetylene, C 2 H 2 (g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is -1301 kJ/mol? Compare this with the answer to Exercise 5.91 and determine...
-
Consider the transition state for a chemical reaction. (a) What is it (define it). (b) Can there be only imminent bond breaking in a transition state? Explain.
-
Leslie Sporting Goods is a locally owned store that specializes in printing team jerseys. The majority of its business comes from orders for various local teams and organizations. While Leslie's...
-
Euclid acquires a 7-year class asset on May 9, 2022, for $153,000 (the only asset acquired during the year). Euclid does not elect immediate expensing under 179. He does not claim any available...
-
Williams & Sons last year reported sales of $10 million, cost of goods sold (COGS) of $8 million, and an inventory turnover ratio of 2. The company is now adopting a new inventory system. If the new...
-
A ceramic manufacturer promised to deliver 25 crates of vases to a Japanese importer under a "CFR" INTERCOM agreement. During transit, however, a large number of vases were broken. The buyer wants to...
-
A company receives $364, of which $23 is for sales tax. The journal entry to record the sale would include a ?
-
Basic capital budgeting problem with accelerated depreciation. Assume the same facts as in Problem 2.1 except that the earnings before depreciation, interest, and taxes is $22,000 per year. (a)...
-
What are the main distinctions between the different schools of legal interpretation?
-
At 350. K, pure toluene and hexane have vapor pressures of 3.57 10 4 Pa and 1.30 10 5 Pa, respectively. a. Calculate the mole fraction of hexane in the liquid mixture that boils at 350. K at a...
-
The partial molar volumes of water and ethanol in a solution with xH 2 O = 0.45 at 25C are 17.0 and 57.5 cm 3 mol 1 , respectively. Calculate the volume change upon mixing sufficient ethanol with...
-
A solution is made up of 222.9 g of ethanol and 130.8 g of H 2 O. If the volume of the solution is 403.4 cm 3 and the partial molar volume of H 2 O is 17.0 cm 3 mol 1 , what is the partial molar...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not
-
Explain: An office building is renting for $10/sf, with 50,000 total leasable square feet. Office buildings in the area are selling for cap rates of 5.5%. What information do you have and what are...

Study smarter with the SolutionInn App


