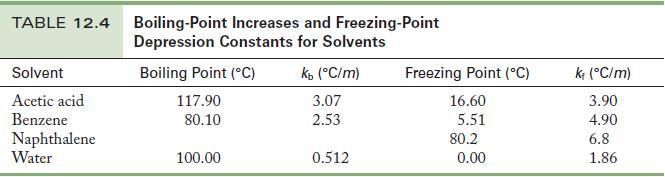
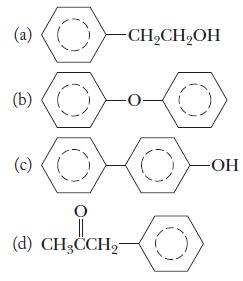
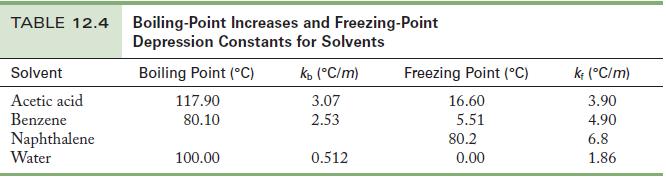
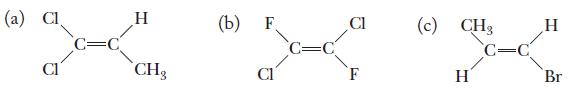
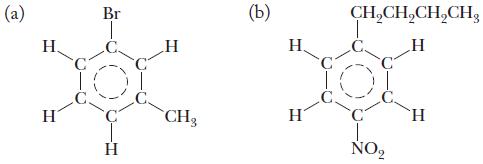
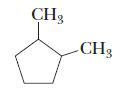
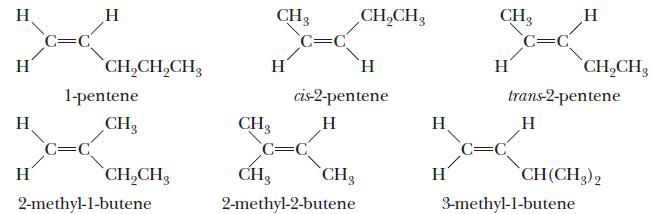
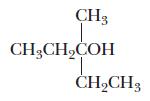
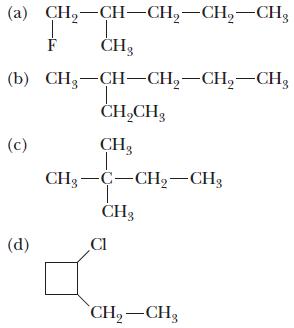
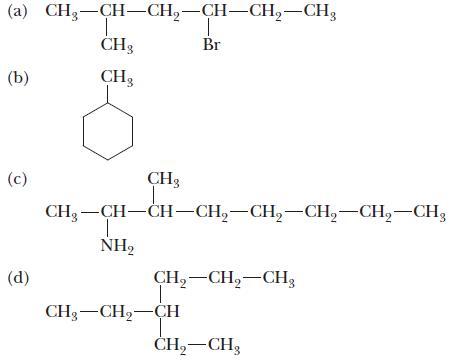
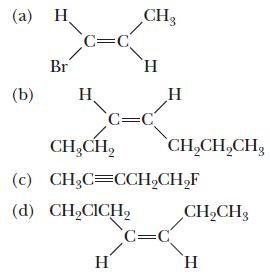
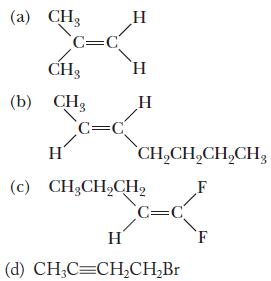
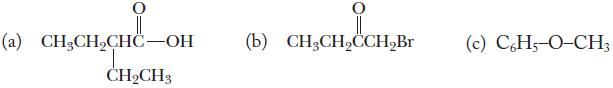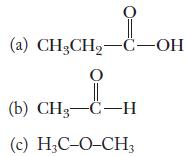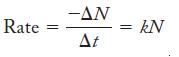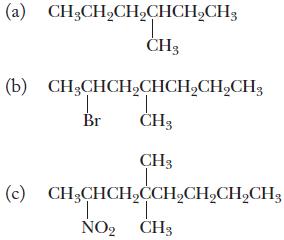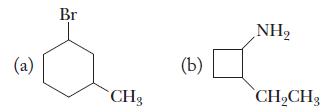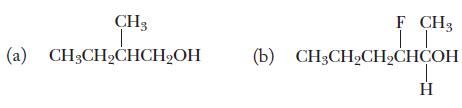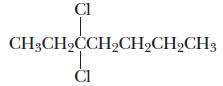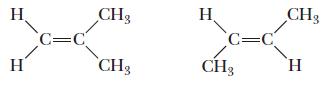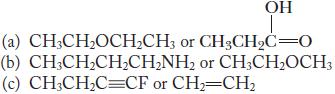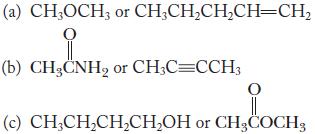Chemistry Principles And Practice 3rd Edition Daniel L. Reger, Scott R. Goode, David W. Ball - Solutions
Discover the ultimate resource for "Chemistry Principles And Practice 3rd Edition" by Daniel L. Reger, Scott R. Goode, and David W. Ball. Access a comprehensive answers key and solutions manual, offering detailed solutions in a convenient PDF format. Our platform provides step-by-step answers to solved problems, covering all chapter solutions and questions and answers from the textbook. Whether you're looking for a test bank, instructor manual, or free download, our online solutions are designed to enhance your understanding and mastery of chemistry concepts. Unlock the full potential of your study with our expertly curated resources.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()