State which compound in each of the following pairs you expect to have the higher boiling point.
Question:
State which compound in each of the following pairs you expect to have the higher boiling point. Explain your answer.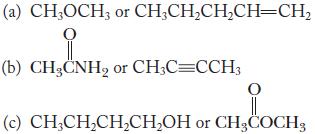
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine which compound in each pair will have the higher boiling point we need to consider the ...View the full answer

Answered By

Joram mutua
I am that writer who gives his best for my student/client. Anything i do, i give my best. I have tutored for the last five years and non of my student has ever failed, they all come back thanking me for the best grades. I have a degree in economics, but i have written academic papers for various disciplines due to top-notch research Skills.In additional, I am a professional copywriter and proofreader.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
State which compound in each of the following pairs you expect to have the higher boiling point. Explain your answer.
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
Good Morning Food, Inc. is using the profitability index (PI) when evaluating projects. You have to find the PI for the company's project, assuming the company's cost of capital is 14.29 percent. The...
-
The step-size parameter plays a critical role in the operation of the LMS algorithm. In this context, discuss the following two issues: (a) Stability. If exceeds a certain critical value, the...
-
Suppose the only goods you consume are wine and roses. On Tuesday, the price of wine goes up, and at the same time your income increases by just enough so that you are equally as happy as you were on...
-
In preparing its income statement for 2017, Parmalane assembles the following information. Sales revenue $500,000 Cost of goods sold 300,000 LO15 Operating expenses 40,000 Loss on discontinued...
-
The unadjusted trial balance for First Class Maids Company, a cleaning service, is as follows: During the 12 months ended December 31, 2016, First Class Maids: a. Used office supplies of $1,900. b....
-
tion 27 Costs incurred to increase the operating efficiency or useful life of a plant asset are referred to as et ered ed out of lag question Select one: a. capital expenditures. b. ordinary repairs...
-
Write the structural formula and name the organic product expected from the acid-catalyzed condensation reaction of CH 3 OH.
-
Draw all the isomers of trichlorobenzene.
-
Neil Armstrong throws a ball down into a crater on the moon. The height s (in feet) of the ball from the bottom of the crater after t seconds is given in the following table: (a) Find the average...
-
The cable supports two cylinders as shown. Cylinders E and F have a mass of 15 kg and 35 kg, respectively. Determine the sag dc and the tension in each segment of the cable. 2 m 2.5 m -2.5m- 2 m dc E...
-
A raft foundation having dimensions of 35 m x 35 m in plan is to be constructed on a deep deposit of sand. Foundation depth and the ground water table are both 5 m below the surface. Unit weight of...
-
Determine the number of 2 X 4 @ 92 5/8" studs needed for the garage in Figures 14.63 and 14.64. The studs are spaced 16 inches on center. Add two studs for each door and corner. Ignore the gable ends...
-
Sketch a cumulative flow diagram that represents the growth and dissipation of a rush hour period at a toll bridge with time-independent capacity. 1) Identify on the diagram: the arrival curve A(t),...
-
Plot the reciprocal lattice for a polycrystalline sample o fa material with a simple tetragonal structure and lattice parameters a = 4.0 A and c = 5.0 A. (Use a two dimensional section through the...
-
A CEO is considering buying an insurance policy to cover possible losses incurred by marketing a new product. If the product is a complete failure, a loss of $800,000 would be incurred; if it is only...
-
Find the equation of the plane passing through the points P 5,4,3 ,Q 4,3,1 and R 1,5,4
-
Will we get an interference pattern in Youngs Experiment (Fig. 9.11) if we replace the source slit S by a single long-filament lightbulb? What would occur if we replaced the slits S 1 and S 2 by...
-
A liquid cell containing an optically active sugar solution has a Jones matrix given by (a) Determine the polarization of the emerging light if the incident beam is a horizontal P-state. (b)...
-
Two linear optical filters have Jones matrices and Identify these filters. 1 eim/4 1 A2 pT/4
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...
-
An investor wants to purchase a zero coupon bond from Timberlake Industries today. The bond will mature in exactly 5.00 years with a redemption value of $1,000. The investor wants a 12.00% annual...

Study smarter with the SolutionInn App


