A 0.500-g sample of a nonvolatile, yellow crystalline solid dissolves in 15.0 g benzene, producing a solution
Question:
A 0.500-g sample of a nonvolatile, yellow crystalline solid dissolves in 15.0 g benzene, producing a solution that freezes at 5.03 °C. Use the data in Table 12.4 to find the molar mass of the yellow solid.
Table 12.4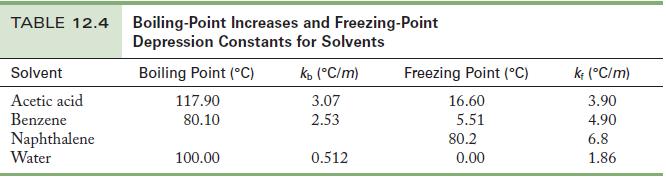
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Heres how to find the molar mass of the yellow solid 1 The freezing point of pure benzene is 551 C f...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A 51.0-mL sample of a gas at 745 torr and 25 C has a mass of 0.262 g. The entire gas sample dissolves in 12.0 g water, forming a solution that freezes at -0.61 C. (a) Calculate the molar mass of the...
-
A 0.350-g sample of a nonvolatile compound dissolves in 12.0 g cyclohexane, producing a solution that freezes at 0.83 C. Cyclohexane has a freezing point of 6.50 C and a freezing-point depression...
-
A 0.325-g sample of dark red crystalline compound dissolves in 12.2 g ethylene dibromide, giving a solution that freezes at 7.97 C. Ethylene dibromide has a normal freezing point of 9.80 C and a...
-
Nevada Department Stores is planning to sell its Spring Valley. Fernley, and Winchester stores. The firm expects to sell each of the three stores for the same, positive cash flow of SD. The firm...
-
(a) A sinusoidal signal, with amplitude of 3.25 volts, is applied to a uniform quantizer of the mid-tread type whose output takes on the values 0, 1, 2, 3 volts. Sketch the waveform of the resulting...
-
You are a theater owner fortunate enough to book a summer box office hit into your single theater. You are now planning the length of its run. Your share of the films projected box office is R = 10w...
-
Ag-Coop is a large farm cooperative with a number of agriculture-related manufacturing and service divisions. As a cooperative, it pays no federal income taxes. It operates a fertilizer plant, which...
-
Paulos Company purchases a controlling interest in Sanjoy Company. Sanjoy had identifiable net assets with a book value of $500,000 and a fair value of $800,000. It was agreed that the total fair...
-
CH 2 On January 1, 2021, Marshall Company acquired 100 percent of the outstanding common stock of Tucker Company. To acquire these shares, Marshall Issued $310,000 in long-term liabilities and 20,000...
-
Name the following compounds. Strategy Follow the steps outlined earlier in the order presented.
-
Explain the difference between distillation and fractional distillation.
-
What are the numbers of observations in each of the four building type categories?
-
Define subjective brightness and brightness adaptation?
-
Write Down The Properties Of Haar Transform?
-
Explain Spatial Filtering?
-
What Is Maximum Filter And Minimum Filter?
-
Name The Categories Of Image Enhancement And Explain?
-
A jar contains four coins: a nickel, a dime, a quarter, and a half-dollar. Three coins are randomly selected from the jar. a. List the simple events in S. b. What is the probability that the...
-
The time to assemble the first unit on a production line is 10 hours. The learning rate is 0.94. Approximately how long will it take for the seventh unit to be assembled? The number of hours needed...
-
Realize the following SM chart using a ROM with a minimum number of inputs, a multiplexer, and a loadable counter (like the 74163). The ROM should generate NST. The multiplexer inputs are selected as...
-
(a) Write Verilog code that describes the following SM chart. Assume that state changes occur on the falling edge of the clock. Use two processes (b) The SM chart is to be implemented using a PLA and...
-
(a) Draw an SM chart that is equivalent to the state graph of Figure 4-46. (b) If the SM chart is implemented using a PLA and three flip-flops (A, B, C), give the PLA-table (state transition table)....
-
What is Apple Companys strategy for success in the marketplace? Does the company rely primarily on customer intimacy, operational excellence, or product leadership? What evidence supports your...
-
Exercise 1 1 - 7 ( Algo ) Net present value and unequal cash flows LO P 3 Gomez is considering a $ 2 1 0 , 0 0 0 investment with the following net cash flows. Gomez requires a 1 2 % return on its...
-
a Campbell Inc. produces and sells outdoor equipment. On July 1, 2011. Campbell issued $40,000,000 a 10-year, 10% bonds at a market (effective) interest rate of 9%, receiving Cash of 548,601,480....

Study smarter with the SolutionInn App


