According to both the Bohr model and the quantummechanical model, the energy of the hydrogen atom can
Question:
According to both the Bohr model and the quantummechanical model, the energy of the hydrogen atom can be calculated from the quantum number n with the following equation: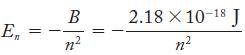
Express the energy, in joules, of the three lowest energy states of the hydrogen atom.
Transcribed Image Text:
En B n² 2.18 X 10-18 J n²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The given formula is E n21810 18 J n 2 Substitute n1 in the formula to find the energy of first ener...View the full answer

Answered By

Nandkumar Ghadge
From my childhood I was fascinated by this world. Knowledge is scattered around that you have to garnish and distribute among others and is to be used for welfare of mankind.
I have 4 years of teaching experience to undergraduate students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
LIST-I contains compounds and LIST-II contains reactions. LIST-I (1) HO (II) (III) (IV) Mg(OH)2 BaCl CaCO3 (P) (Q) (R) LIST-II Mg(HCO3) + Ca(OH) BaO,+H,SO4 Ca(OH) + MgCl BaO, +HCI (T) Ca(HCO3)2 +...
-
The Bohr model of the hydrogen atom states that the single electron can exist only in certain allowed orbits around the proton. The radius of each Bohr orbit is r = n2(0.052 9 nm) where n = 1, 2, 3,...
-
Which one of the following statements is false? (a) The orbits in the Bohr model have precise sizes, whereas in the quantum mechanical picture of the hydrogen atom they do not. (b) In the absence of...
-
Data-2-Go manufactures and sells flash drives. The company produces only when it receives orders and, therefore, has no inventories. The following information is available for the current month:...
-
A uniform rod of mass M = 20 kg and length L = 5 m is bent into a semicircle. What is the gravitational force exerted by the rod on a point mass m = 0.1 kg located at the center of the circular arc?
-
If the velocity of a particle is zero, can its acceleration be nonzero? Explain.
-
how researchers used nested models to examine how race affects grades
-
Allocating resources can be a political and an ad hoc activity in firms that do not use strategic management. Why is this true? Does adopting strategic management ensure easy resource allocation? Why?
-
PART III - CLOSING ENTRIES (10 points) Below is a partial listing of accounts in the general ledger of Denton Co. Instructions: Place an X in the appropriate column to designate whether the account...
-
Many computer manufacturers now include tools or simulators that allow you to measure the instruction set usage of a user program. Among the methods in use are machine simulation, hardware-supported...
-
Find the uncertainty in the position (in m) of a 650-kg automobile that is moving at 55 mph if the speed is known to within 1 mile/hr. Is this uncertainty in position significant?
-
The energy expression given for the allowed states in the hydrogen atom, -2.18 10 -18 J/n 2 , refers to a single atom. Express the energy of the allowed states (in kJ/mol).
-
Which is a character literal, 'B' or "B"?
-
Home Base, Incorporated reports the following production cost information: Units produced 97,000 units Units sold 92,000 units Ending finished goods inventory 5,000 units Direct labor $17 per unit...
-
About New York City public sector finance. The other way is to delineate the problem. We should use data to show a problem, and then analyze the environment in which budgeting takes place to suggest,...
-
From a survey a company has determined that 23% of its customers are classified as "advocates" , 68% as "passives" and the remainder as "detractors" . Research suggests that during a year 15% of the...
-
The following are the transactions of Spotlighter, Incorporated, for the month of January. a. Borrowed $3,940 from a local bank on a note due in six months. b. Received $4,630 cash from investors and...
-
1. What are the deeper problems that plague in different forms it takes throughout the world according to the authors? Please, briefly explain. 2. Why was Joseph Schumpeter a pessimist about the...
-
A 1-m-square vertical plate is heated to 300oC and placed in room air at 25oC. Calculate the heat loss from one side of the plate.
-
Use integration by parts to evaluate the following. Check your answer by taking the derivative. x2e-xdx
-
Calculate the rotational partition function for the interhalogen compound F 35 Cl (B = 0.516 cm 1 ) at 298 K.
-
Calculate the rotational partition function for 35 Cl 2 (B 0.244 cm -1 ) at 298 K.
-
For which of the following diatomic molecules is the high-temperature expression for the rotational partition function valid at 40. K? a. DBr (B = 4.24 cm 1 ) b. DI (B = 3.25 cm 1 ) c. CsI (B =...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...
-
The rate of return on Cherry Jalopies, Inc., stock over the last five years was 14 percent, 11 percent, 4 percent, 3 percent, and 7 percent. What is the geometric return for Cherry Jalopies, Inc.?
-
U.S. GAAP specifies all of the following characteristics of variable interest entities except: A. Equity holders hold less than 5% of the entitys voting stock. B. Equity holders do not have voting...

Study smarter with the SolutionInn App


