Calculate the energy necessary to boil 100.0 g carbon disulfide, CS 2 , at its normal boiling
Question:
Calculate the energy necessary to boil 100.0 g carbon disulfide, CS2, at its normal boiling point.
Strategy
Determine the number of moles of carbon disulfide; then use the enthalpy of vaporization from Table 11.3 as a conversion factor to determine the energy needed.
Table 11.3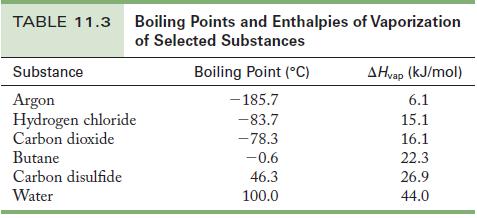
Transcribed Image Text:
TABLE 11.3 Boiling Points and Enthalpies of Vaporization of Selected Substances Substance Argon Hydrogen chloride Carbon dioxide Butane Carbon disulfide Water Boiling Point (C) -185.7 -83.7 -78.3 -0.6 46.3 100.0 AHvap (kJ/mol) 6.1 15.1 16.1 22.3 26.9 44.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The molar mass of CS 2 is 7613 gmol The number o...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Table B.2 of Appendix B provides parameters for an equation that gives P sat as a function of T for a number of pure compounds. For one of them, determine the heat of vaporization at its normal...
-
The enthalpy of vaporization of methanol is 35.27 k] mol-I at its normal boiling point of 64.1oC. Calculate (a) The entropy of vaporization of methanol at this temperature and (b) The entropy change...
-
The normal boiling point of Br2(l) is 58.8C, and its molar enthalpy of vaporization is Hvap = 29.6 kJ/mol. (a) When Br2(l) boils at its normal boiling point, does its entropy increase or decrease?...
-
Determine which of the following statement(s) will always be true. It is possible that more than one statement is true. Only write down the letters of the statements that are true. n +1 2.1 The...
-
In the circuit in figure, the switch moves from position 1 to position 2 at t = 0. Use Laplace transforms to find v(t) for t >0. 6 t= 0 12 v (+ 100 pF (1)a 6 kn
-
The following items, in alphabetical order, are available from the records of Quinn Corporation as of December 31, 2010 and 2009: Required 1. Calculate the following as of December 31, 2010, and...
-
Independent random samples, each containing 800 observations, were selected from two binomial populations. The samples from populations 1 and 2 produced 320 and 400 successes, respectively. a. Test...
-
Find the position of the center of mass of the system of the sun and Jupiter. (Since Jupiter is more massive than the rest of the planets combined, this is essentially the position of the center of...
-
Answer the following questions using the information below: Mayan Potters manufactures two sizes of ceramic paperweights, regular and jumbo. The following information applies to their expectations...
-
Aspartame is a compound that is 200 times sweeter than sugar and is used extensively (under the trade name NutraSweet) in diet soft drinks. The skeleton structure of the atoms in aspartame is H-0. ....
-
Identify the intermolecular forces of attraction, and predict which substance of each pair has the stronger forces of attraction. Strategy All of the molecules will have London dispersion forces. We...
-
Compare the traditional organization with a TQM organization.
-
how is lateral force(fy) determined from this data Tyre Responses 1 1 1 1 1 1.3 1.3 1.3 1.3 1.3 1.6 1.55 1.45 1.27 1.1 Fz (N) 0 400 800 1200 1500 Slip Angle (deg) Fy1 (N) Fy2 (N) Fy3 (N) 0.0 0 0 0.5...
-
(13%) Problem 8: A wire is oscillated to create a wave of the form y(x,t) = Asin(x - 30t) == The wave is reflected from a fixed end producing a reflection of the form y2(x,t) = A sin(x + 30t) The two...
-
Using the definitions of even integer and odd integer, give a proof by contraposition that this statement is true for all integers n: If 5n+3 is even, then n is odd.
-
7. Design the formwork for a wall 8-ft (2.44-m) high to be poured at the rate of 5 ft/h (1.53 m/h) at a temperature of 77F (25C). The concrete mixture will use Type I cement without retarders and is...
-
tempt in Progress The City of Minden entered into the following transactions during the year 2026. 1. A bond issue was authorized by vote to provide funds for the construction of a new municipal...
-
Using the reaction HPO24- + 2H+ + 2e- HPO2-3 = H2O E = - 0.234 V and acid dissociation constants from Appendix G, calculate E for the reaction H2PO4- + H+ + 2e- HPO2-3 + H2O
-
Difference between truncate & delete
-
The inner ring A has an inner radius r 1 and outer radius r 2 . The outer ring B has an inner radius r 3 and an outer radius r 4 , and r 2 > r 3 . If the outer ring is heated and then fitted over the...
-
The ring, having the dimensions shown, is placed over a flexible membrane which is pumped up with a pressure p. Determine the change in the inner radius of the ring after this pressure is applied....
-
An A-36-steel hoop has an inner diameter of 23.99 in., thickness of 0.25 in., and width of 1 in. If it and the 24-in.-diameter rigid cylinder have a temperature of 65° F, determine the...
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


