Calculate the pH of 0.20 M pyridine. Use Table 15.8 to find K b . Table 15.8
Question:
Calculate the pH of 0.20 M pyridine. Use Table 15.8 to find Kb.
Table 15.8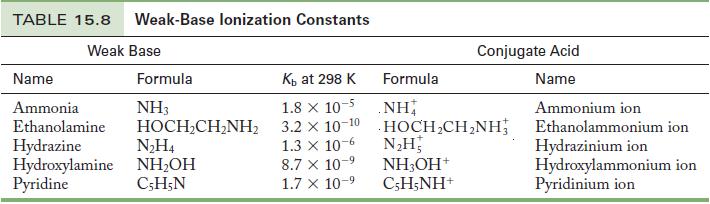
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)

Answered By

Deborah Joseph
My experience has a tutor has helped me with learning and relearning. You learn everyday actually and there are changes that are made to the curriculum every time so being a tutor has helped in keeping me updated about the present curriculum and all.
I have also been able to help over 100 students achieve better grades particularly in the categories of Math and Biology both in their internal examinations and external examinations.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate the pH of each of the following solutions (Ka and Kb values are given in Appendix D): (a) 0.095 M propionic acid (C2H5COOH), (b) 0.100 M hydrogen chromate ion (HCrO4-, (c) 0.120 M pyridine...
-
Calculate the pH of a solution made by mixing 0.60 L of 0.10 M NH4Cl with 0.50 L of 0.10 M NaOH. Kb for NH3 is 1.8 10-5.
-
The Kb for NH3 is 1.8 10-5 at 25C. Calculate the pH of a buffer solution made by mixing 65.1 mL of 0.142 M NH3 with 39.2 mL of 0.172 M NH4Cl at 25C. Assume that the volumes of the solutions are...
-
Norma received a deficiency letter from the Internal Revenue Service. She appealed to the Independent Office of Appeals, and her appeal was denied. Now she wants to take the government to court....
-
The following 1H NMR absorptions were obtained on a spectrometer operating at 200MHz and are given in hertz downfield from the TMS standard. Convert the absorptions to units. (a) 436 Hz (b) 956 Hz...
-
Why should younger employees (those in their 20s and 30s) care about retirement benefits?
-
DE 19-1 Your roommate, who plans to specialize in international business, is considering whether to enroll in the second principles of accounting course. She says, "I don't want to be an accountant,...
-
The Fanta Company presents you with the following account balances taken from its December 31, 2007 adjusted trial balance: Inventory, January 1, 2007 ............. $ 43,000 Selling expenses...
-
company specific average, trend analysis Quarter Ending Mar 14 June 14 Sept 14 Dec 14 Mar 15 June 15 Sept 15 Dec 13 Mar 16 June 16 Sept 16 Dec 16 Snap Inc. Company Metrics Daily Active Users Revenue...
-
Calculate K a for the ammonium ion at 25 C.
-
Calculate the percentage ionized in solutions that are 0.0100 and 0.0010 M naphthol.
-
King Mattresses sells both mattress sets and bed frames. Last quarter total sales were $50,000 for mattress sets and $25,000 for bed frames. ROI was 10 percent for both divisions, while asset...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Identifying Binomial Distributions. Determine whether the given procedure results in a binomial distribution or a distribution that can be treated as binomial (by applying the 5% guideline for...
-
Case 6: TOMS Shoes in 2016: An Ongoing Dedication to Social Responsibility, by Margaret A. Peteraf, Sean Zhand, and Meghan L. Cooney (page C-57) Read the case and then respond to the case questions...
-
Quatro Co. issues bonds dated January 1, 2019, with a par value of $740,000. The bonds' annual contract rate is 13%, and interest is paid semiannually on June 30 and December 31. The bonds mature in...
-
Wildcat Mining wants to know the appropriate discount rate to use in their capital budgeting decision making process. Based on the following data, what is the weighted average cost of capital the CFO...
-
For each of the intermetallic compounds shown in Figure 12.17 determine the number of each type of atom in the unit cell. Do your answers correspond to ratios expected from the empirical formulas:...
-
Juanita owns a home in Richardson, TX. She purchases a Homeowners Policy (HO-3) from Farm State Ins. Co. The policy provides $100,000 in liability coverage (coverage E) and $5,000 in Med Pay coverage...
-
Fuchsin is a strong (aniline) dye, which in solution with alcohol has a deep red color. It appears red because it absorbs the green component of the spectrum. (As you might expect, the surfaces of...
-
Take Eq. (3.71) and check out the units to make sure that they agree on both sides. Nq? n(m) = 1 + o me (3.71) lWj w @j
-
The resonant frequency of lead glass is in the UV fairly near the visible, whereas that for fused silica is far into the UV. Use the dispersion equation to make a rough sketch of n versus for the...
-
Mediocre Company has sales of $120,000, fixed expenses of $24,000, and a net income of $12,000. If sales rose 10%, the new net income would be: Question 18 options: $16,800 $36,000 $13,200 $15,600
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...

Study smarter with the SolutionInn App


