Calculate the standard enthalpy of formation for glucose, C 6 H 12 O 6 (s), from the
Question:
Calculate the standard enthalpy of formation for glucose, C6H12O6(s), from the following information. A calorimetry experiment shows that the enthalpy of combustion of 1 mol glucose to form carbon dioxide and water at 298.15 K is -2807.8 kJ. Use the data in Table 5.2 for the standard enthalpies of formation of carbon dioxide and water.
Strategy
ΔH for the reaction was measured and ![]() for the products are in Table 5.2. The enthalpy of formation of oxygen gas is zero, so we can calculate
for the products are in Table 5.2. The enthalpy of formation of oxygen gas is zero, so we can calculate ![]() for glucose.
for glucose.
Table 5.2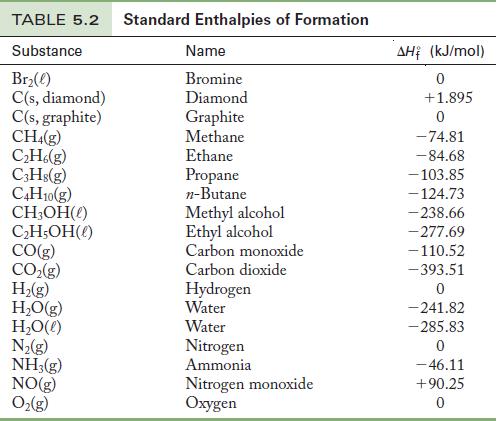
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





