Calculate the standard free energy change for the reaction Strategy Use Table 18.1 to determine the cell
Question:
Calculate the standard free energy change for the reaction![]()
Strategy Use Table 18.1 to determine the cell potential; then use Equation 18.2 to calculate the ΔG°. You will also have to determine the number of moles of electrons, n, that are transferred in this reaction.
Equation 18.2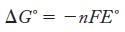
Table 18.1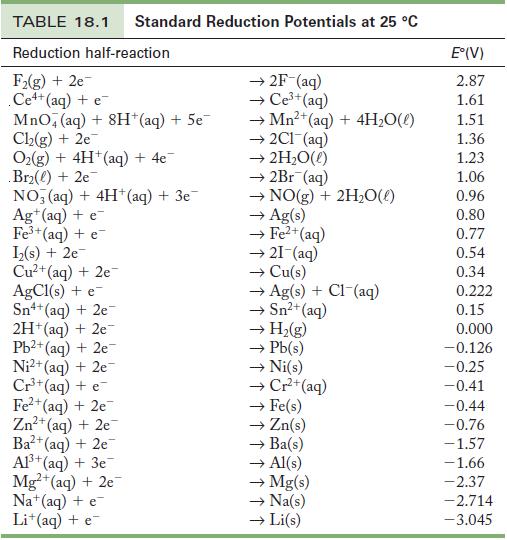
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
From the table of standard reduction potentials the t...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Suppose that we wish to study the possible galvanic corrosion between zinc and chromium, so we set up the following cell: What is the chemical reaction that takes place, and what is the standard free...
-
Calculate the standard free energy change for the dissociation of HEPES.
-
The standard free energy change for the hydrolysis of ATP was given in Problem 91. In a particular cell, the concentrations of ATP, ADP, and P i are 0.0031 M, 0.0014 M, and 0.0048 M, respectively....
-
____, one-way ANOVA compares the means of three or more independent samples.
-
Referring to the distribution of the spectral transmissivity of low iron glass (Figure), describe briefly what is meant by the "greenhouse effect." That is, how does the glass influence energy...
-
"Melinda!" bellowed Toran to the company's HR specialist, "I've got a problem, and you've got to solve it. I can't get people in this plant to work together as a team. As if I don't have enough...
-
What are the principal sources of secondary research? What are the advantages and disadvantages of secondary research?
-
Texon Co. manufactures and sells three products: product 1, product 2, and product 3. Their unit sales prices are product 1, $40; product 2, $30; and product 3, $14. The per unit variable costs to...
-
I do not need EXCEL solution. Please don't give any solution using EXCEL. Counterparty A and Counterparty B agree to swap $50 million loans. Counterparty A currently pays LIBOR + 75 basis points and...
-
What is the equilibrium constant for the following reaction? Strategy Determine the number of electrons transferred, then use Equation 18.4. Equation 18.4
-
Use Table 18.1 to (a) List metals that are and are not oxidized by H + (aq) under standard conditions. (b) Find an oxidizing agent that will oxidize copper metal. Strategy Consult Table 18.1 as an...
-
Match the function with its graph using horizontal asymptotes as an aid. [The graphs are labeled (a), (b), (c), (d), (e), and (f).] (a) (b) (c) (d) (e) (f) -2 -1 3 1 y 1 2 X
-
In the global discourse on healthcare, the United States and England stand out as two contrasting models, each providing a distinct approach to addressing the challenges of cost , access, and...
-
2.A. Using the quotes below, answer the following questions. Exchange rate Bid Ask In New York, USD/EUR 1.2267 1.2875 In London, USD/GBP 1.6555 1.7334 2.A1. Calculate the EUR/GBP cross exchange...
-
Question 43 Part B Q1ii 20 points Save A a) A property is currently leased for $100,000 p.a. with fully recoverable outgoings. The lease has 3 years to run on the current (fixed) rent. The market...
-
Define HIPPA? What is the purpose of HIPPA? What are the 4 main rules of HIPPA?
-
Accounting for Inventories" Please respond to the following: As a Financial Accountant,determine the best type of income statement a retailer should use.Defend your suggestion. Analyze inventory...
-
Does the reactivity of a metal correlate with its first ionization energy? Explain.
-
If the cylinder described in Problem 21.3 were initially heated to 500F, how long would it take for the center of the cylinder to cool to 240F if it were constructed of a. Copper? b. Brass? c. Nickel?
-
Repeat Problem 6.100 if the tank is sealed and a pressure of 35 kPa(gage) is above the water in the tank. Repeat Problem Compute the time required to reduce the depth in the tank shown in Fig. 6.14...
-
Repeat Problem 6.96 if the tank is sealed and a pressure of 20 kPa(gage) is above the water in the tank. Repeat Problem Compute the time required to empty the tank shown in Fig. 6.14 if the original...
-
Repeat Problem 6.101 if the tank is sealed and a pressure of 2.8 psig is above the water in the tank. Repeat Problem Compute the time required to reduce the depth in the tank shown in Fig. 6.14 by...
-
A contractor constructed a house for resale, which was sold immediately. For tax purposes, this is an example of A) capital income. B) business income. C) other income. D) property income.
-
You invest $100 in a risky asset with an expected rate of return of 0.12 and a standard deviation of 0.15 and a T-bill with a rate of return of 0.05. What percentages of your money must be invested...
-
Nanometrics, Inc., has a beta of 3.43. If the market return is expected to be 13.50 percent and the risk-free rate is 7.00 percent, what is Nanometrics required return? (Round your answer to 2...

Study smarter with the SolutionInn App


