Calculate the vapor pressure of each of the following at the given temperature. For each reaction, an
Question:
Calculate the vapor pressure of each of the following at the given temperature.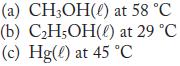
For each reaction, an equilibrium constant at 298 K is given. Calculate ΔG ° for each reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Here are the calculated G values for each reaction ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
City jail database
-
Solve each formula for the specified variable. (Leave in the answers as needed.) P = kl 00 g for g
-
1. Explain why corrective action should be taken as soon as possible and practical following an infraction. 2. Why is it necessary to have a completely documented history leading up to an employee's...
-
Jin, the recruitment manager at Randents Inc., reviews the performance of his team members on a monthly basis. Based on the results of his monthly reviews, he decides to conduct daily reviews to...
-
For a prescribed wavelength A, measurement of the spectral intensity I.c (, T) = I, b of radiation emitted by a diffuse surface may be used to determine the surface temperature, if the spectral...
-
1. Write a decision statement for Raising Canes. 2. Write corresponding research objectives and research questions. 3. What role would a proposal play in assisting this research effort and in...
-
10. How is the combined cost of goods sold affected by unrealized profit in (a) the beginning inventory of the subsidiary and (b) the ending inventory of the subsidiary?
-
How do free cash flows and the weighted average cost of capital interact to determine a firms value? MINI CASE Assume that you recently graduated with a degree in finance and have just reported to...
-
Kintel, Inc., wants to raise $$1 million by issuing six-year zero-coupon bonds with a face value of $$1,000. Their investment banker informs them that investors would use an 11.4 percent discount...
-
A 220-ft 3 sample of gas at standard temperature and pressure is compressed into a cylinder, where it exerts pressure of 2000 psi. Calculate the work (inJ) performed when this gas expands...
-
Calculate the vapor pressure of each of the following at the given temperature. For each reaction, an equilibrium constant at 298 K is given. Calculate G for each reaction.
-
Jamison Cruise Lines, Inc., reported the following income statement for the year ended December 31, 2012: Requirements 1. Were Jamisons discontinued operations more like an expense or revenue? How...
-
Question 2 of 6 When can XWFs approve expenses on behalf of Google? If a Director has approved it. If your employer has approved it. XWFs cannot approve expenses on behalf of Google. If it's under...
-
Does the following table represent a valid discrete probability distribution? x 1 2 3 4 5 P ( X = x ) 0.11 0.06 0.25 0.41 0.51
-
Semester Two Practice Examinations, 2022 Question 1. [10 marks] Suppose X and Y have the joint probability mass function x 0 1 2 0 CO 0.175 0.105 1 0.18 0.075 C1 where CO and C are real numbers such...
-
Problem 7 (40 pts) Ethylene glycol (p=1096 Kg/m, C=2505 J/KgK, v=6.9x10-6 m/s, Pr=73.5) is pumped through a pipeline of diameter D=0.4 m that runs across a lake L=200 m wide. The bulk velocity and...
-
The number of fully formed apples on 100 plants was counted with the following results: 2 plants had 0 apples 5 plants had 1 apple 7 plants had 2 apples 11 plants had 3 apples 18 plants had 4 apples...
-
In the chemical process called electron transfer, an electron is transferred from one atom or molecule to another. (We will talk about electron transfer extensively in Chapter 20.) A simple electron...
-
Data 9.2 on page 540 introduces the dataset Cereal, which includes information on the number of grams of fiber in a serving for 30 different breakfast cereals. The cereals come from three different...
-
A large chipper/shredder is to be designed for use by commercial tree trimming companies. It would be mounted on a trailer to pull behind a large truck. The rotating blades of the unit protrude from...
-
A hot tub is to have 40 outlets that are each 8 mm in diameter with water exiting at 7 m/s. Treating each of the outlets as if they are at the surface of the water and exit into atmospheric pressure,...
-
A creek runs through a certain part of a campus where the water is falling about 2.5 m over a distance of just 8 m, and the creek before and after the fall is about 3 m wide. The sustainability club...
-
Smile Company makes baked goods. The budgeted sales are $620,000, budgeted variable costs are $260,400, and budgeted fixed costs are $237,800. What is the budgeted operating income?
-
Analysis of a replacement project At times firms will need to decide if they want to continue to use their current equipment or replace the equipment with newer equipment. In this case, the company...
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...

Study smarter with the SolutionInn App


