Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
Question:
Complete and balance each half-reaction in acid solution,
and identify it as an oxidation or a reduction.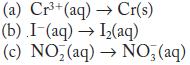
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To complete and balance each halfreaction in acid sol...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
-
Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
-
(a) Nitrous acid reacts with hydrazine in acidic solution to form hydrazoic acid, HN 3 . Write the chemical equation and determine the mass of hydrazoic acid that can be produced from 15.0 g of...
-
1 YOUR NAME: 2 Asset # 3 Asset name: 4 Date acquired: 5 Cost: 6 Depreciation method: 7 Salvage (residual) value: 8 Estimated useful life (years): 9 222 Computer 1/1/2018 $50,000 Straight Line (SL)...
-
To simulate materials processing under the microgravity conditions of space, a niobium sphere of diameter 3 mm is levitated by an acoustical technique in a vacuum chamber. Initially the sphere is at...
-
Define the purpose of a code of ethics, and write a brief code that would be suitable for a small business.
-
Kellogg Company Kellogg Company in its 2004 Annual Report in Note 1Accounting Policies made the following comment about its accounting for employee stock options and other stock-based compensation....
-
Banks earn money by borrowing from depositors at low interest rates and lending to individuals and businesses at high interest rates. As banks grow, they split into functional divisions that either...
-
Robert Martins gross earnings for the week ending on December 27 amount to $2,200. His cumulative gross earnings for the year up through his last pay date amount to $89,300. Using the table below,...
-
Write balanced equations for the following half reactions. Specify whether each is an oxidation or reduction.
-
Balance the following reactions, and specify which species is oxidized and which is reduced. (a) Na + Hg 2 Cl 2 NaCl + Hg (b) HCl + Zn ZnCl 2 + H 2 (c) H 2 + CO 2 CO + H 2 O
-
Assuming that air is composed of O 2 , O, N 2 , N, and NO, and that only O 2 and N 2 are present in significant amounts at room temperature in the ratio of 3.76 moles of N 2 per mole of O 2 ,...
-
How do you demonstrate resilience as a leader during times of crisis or uncertainty, and what steps do you take to bolster your team's resilience ?
-
What would you do if it becomes clear to you that the potential successor you were grooming is not going to make the grade as a supervisor? What are your next steps? Do you think this grooming is...
-
How do services and products differ? What kind of decisions do companies make regarding products and services? Why are brands important to marketers? How do marketing strategies change during the...
-
What leadership principles do you feel you possess that are important for APRNs to exhibit? What principles do you need to explore to be more confident in performing? Which leadership style do you...
-
Question 1- Where do you go in the Courier to find out your amount of leftover inventory for a specific product last round? Based on the production tab of the worksheet I gave you; how do you use...
-
Barium azide is 62.04% Ba and 37.96% N. Each azide ion has a net charge of 1-. (a) Determine the chemical formula of the azide ion. (b) Write three resonance structures for the azide ion. (c) Which...
-
Use this circle graph to answer following Exercises. 1. What fraction of areas maintained by the National Park Service are designated as National Recreation Areas? 2. What fraction of areas...
-
Your friends car has broken down, and you volunteer to push it to the nearest repair shop, which is 2.0 km away. You carefully move your car so that the bumpers of the two cars are in contact and...
-
A tall strongman of mass m = 95 kg stands upon a scale while at the same time pushing on the ceiling in a small room. Draw a free-body diagram of the strongman (Fig. P3.34) and indicate all normal...
-
A bodybuilder configures a leg-press apparatus (Fig. P3.35) to a resistance of 1000 N (about 230 lb). She pushes the weight to her full extension and comes to rest. (a) What is the normal force on...
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...

Study smarter with the SolutionInn App


