Determine the molarity of an aqueous solution that is 37.2% in HCl. The density of this solution
Question:
Determine the molarity of an aqueous solution that is 37.2% in HCl. The density of this solution is 1.034 g/mL.
Strategy
Write the concentrations as fractions.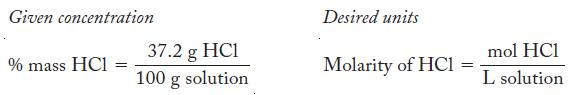
The units in the numerator of percentage composition can be easily converted to moles, and we can use the density of the solution to convert from grams of solution to volume of solution.
Transcribed Image Text:
Given concentration % mass HC1 = 37.2 g HC1 100 g solution Desired units Molarity of HC1 = mol HCl L solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
The molar mass of HCl is 3646 gmol Converting the a...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
I Jose and Laura go to a rock concert. Jose doesn't feel well and wants to go home, but Laura cannot hear Jose over the music. What component of communication are Jose and Laura experiencing? O...
-
Consider a 27-year bond with $1,000 face value that pays a 8.00% coupon on an annual basis and has a yield-to-maturity of 7.00%. What is the approximate percentage change in the price of bond if...
-
Alleghany Community College operates four departments. The square footage used by each department is shown below. Alleghany's annual building rental cost is $320,000 What amount of rent expense that...
-
Find the impedance ZL for maximum average power transfer and the value of the maximum average power transferred to ZL for the circuit shown in figure. 12/0 V j1n 310 10 > 21 ZL -j1n
-
1. What is the goal of Superfund? 2. Who polluted this site? 3. Then why cant Aviall seek contribution from Cooper if Cooper is also responsible for the pollution?
-
Why, in practice, would borrowing by the business to raise cash to pay a dividend not be economically equivalent to borrowing by the individual shareholders to provide themselves with cash? (Note...
-
Each of the following compounds is characterized by a 1H NMR spectrum that consists of only a single peak having the chemical shift indicated. Identify each compound. (a) C8H18; 0.9 ppm (f) C2H3Cl3;...
-
In order to send your first child to law school when the time comes you want to accumulate $40,000 at the end of 18 years. Assuming that your savings account will pay 6% compounded annually how much...
-
Predict the solvent in which the given compound is more soluble, and justify your prediction. Strategy Consider the types of intermolecular interactions in the solute and the solvents. Solubility...
-
Find the molarity of a 7.85% aqueous ammonia solution that has a density of 0.965 g/mL.
-
Let \(\mu>A / C\) and consider the following optimization problem: \[\begin{equation*}\max _{w \in \Delta_{N}} \frac{w^{\top} e-\mu}{\sqrt{w^{\top} V w}} \tag{3.56}\end{equation*}\] corresponding to...
-
If f ( x ) = ( 1 3 - In ( x ) ) ^ 8 , determine f ' ( 1 ) .
-
1. ThestocksAandBhavethefollowingdistributionsofreturns. A B Probability State1 3 4 0.2 State2 5 2 0.3 State3 4 8 0.2 State4 6 5 0.1 State5 6 1 0.2 2....
-
Define nested designs. Explain why the nested designs are important.
-
3 x y 3 + x y = l n ( x ) solve for d y d x
-
Let ln ( xy ) + y ^ 8 = x ^ 7 + 2 . Find dy / dx .
-
(a) Following the example of ammonia in Section 7-5, write the equilibria and charge and mass balances needed to find the composition of 0.01 M sodium acetate, which you should abbreviate as Na+A-....
-
What is removed during each of the three stages of wastewater treatment: primary, secondary, and tertiary? During which state would you expect items to be recovered that were accidentally flushed,...
-
Determine the displacement at D and the slope at D. Assume A is a fixed support,Bis a pin, and C is a roller. Use the conjugate-beam method. |B Ic |A 12 ft 12 ft 12 ft
-
Determine the displacement at D and the slope at D. Assume A is a fixed support,Bis a pin, and C is a roller. Use the moment-area theorems. 6 k D B |A - 12 ft 12 ft 12 ft-
-
Determine the displacement at C. Assume A is a fixed support,Bis a pin, and Dis a roller. EI is constant. Use the conjugate-beam method. 25 kN -3 m -3 m 3 m
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


