Explain why the lattice energy of Na 2 O is considerably greater than that of NaF. Strategy
Question:
Explain why the lattice energy of Na2O is considerably greater than that of NaF.
Strategy
Lattice energies are evaluated by using the relationship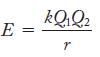
In general, the changes that can take place with the Q terms are more important than small changes in r.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





