For each reaction, an equilibrium constant at 298 K is given. Calculate G for each reaction.
Question:
For each reaction, an equilibrium constant at 298 K is given. Calculate ΔG ° for each reaction.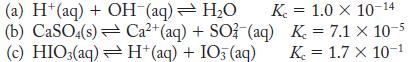
Aassume that ΔH ° and ΔS ° do not change with temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a G 799 ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Suppose you have an exothermic reaction with H = -15 kJ and a S of -150 J/K. Calculate G and K eq at 10, 100, and 1000 K. For each reaction, an equilibrium constant at 298 K is given. Calculate G ...
-
Suppose you have an exothermic reaction with H = -15 kJ and a S of -150 J/K. Calculate G and K eq at 10, 100, and 1000 K. For each reaction, an equilibrium constant at 298 K is given. Calculate G ...
-
Suppose you have an endothermic reaction with H = +15 kJ and a S of -150 J/K. Calculate G and K eq at 10, 100, and 1000 K. For each reaction, an equilibrium constant at 298 K is given. Calculate G...
-
The toroid of FIGURE P29.55 is a coil of wire wrapped around a doughnut-shaped ring (a torus). Toroidal magnetic fields are used to confine fusion plasmas. a. From symmetry, what must be the shape of...
-
An electrically powered, ring-shaped radiant heating element is maintained at a temperature of Th = 3000 K and is used in a manufacturing process to heat a small part having a surface area of Ap =...
-
Design the coding for this portion of the questionnaire. Assume that the data from previous pages of the questionnaire will follow these data. Some years ago the U.S. Department of the Interior...
-
E 5-2 [Based on AICPA] General problems 1. Pop, Inc., owns 80 percent of Son, Inc. During 2016, Pop sold goods with a 40 percent gross profit to Son. Son sold all of these goods in 2016. For 2016...
-
A small motor manufacturer makes two types of motor, models A and B. The assembly process for each is similar in that both require a certain amount of wiring, drilling, and assembly. Each model A...
-
3.1. Xolani consults with you regarding his employment contract. He complains that it is not detailed as it does not deal with his entitlements in regard to leave. Write a response to Xolani in which...
-
Aassume that H and S do not change with temperature.
-
Calculate the normal boiling point of nitromethane (CH 3 NO 2 ). Use the thermodynamic data in Appendix G. Compare your answer with the experimentally measured boiling point. Aassume that H and S ...
-
The 1 H NMR spectrum of a compound with molecular formula C 7 H 15 C l exhibits two signals with relative integration 2 : 3. Propose a structure for this compound.
-
1. A large group of students were asked what their favorite soft drink is. Below is the probability distribution for a student chosen at random liking a particular soft drink. Drink: Choka Kola CR...
-
Task: Identify a local (within 50km of North Bay) business and answer the following questions: Name of Business: 1. Is the business independent or is it a chain? What is one advantage of this...
-
What questions would you like to ask of Cassie to better understand any factors that may be affecting Sasha at this time? Growing sunflowers It's now week 6 into the growing sunflowers project. Your...
-
n rope is fixed to a wall and attached to the block such that the rope is parallel to the surface of the wedge. The 12 points) Consider the situation in the figure where a square block (mi) sits...
-
Consider the iso-electronic ions Cl- and K+. (a) Which ion is smaller? (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute nothing to the...
-
Under what conditions is the following SQL statement valid?
-
Water at 75C is flowing in a standard hydraulic copper tube, 15 mm OD 1.2 mm wall, at a rate of 12.9 L/min. Calculate the pressure difference between two points 45 m apart if the tube is horizontal.
-
Fuel oil is flowing in a 4-in Schedule 40 steel pipe at the maximum rate for which the flow is laminar. If the oil has a specific gravity of 0.895 and a dynamic viscosity of 8.3 10 -4 lb - s/ft 2 ,...
-
Use PIPE-FLO to model a straight horizontal run of 100 ft of 1-in Schedule 40 pipe carrying 20 gal/min of 75F water from a tank with a water level of 25 ft. Display the calculated pressure drop in...
-
What is the present value of $500 invested each year for 10 years at a rate of 5%?
-
GL1203 - Based on Problem 12-6A Golden Company LO P2, P3 Golden Corp.'s current year income statement, comparative balance sheets, and additional information follow. For the year, (1) all sales are...
-
A project with an initial cost of $27,950 is expected to generate cash flows of $6,800, $8,900, $9,200, $8,100, and $7,600 over each of the next five years, respectively. What is the project's...

Study smarter with the SolutionInn App


